Abstract
Stimuli-responsive gels have been actively studied and applied to a variety of devices such as drug carriers, artificial muscles and actuators. In this research study, calcium alginate gels containing hydroxyapatite were prepared in one step by adding a diammonium hydrogenphosphate–1% alginate solution to a calcium chloride solution and evaluated for pH dependence and electrochemical properties as stimuli-responsive gels. When the composite gel was immersed in a solution of pH 2–10 or subjected to an applied voltage, the composite gel with a high content of hydroxyapatite tended to maintain its volume. The composite gels immersed in a pH indicator solution changed color with an applied voltage. As a result, it was revealed that alteration of the pH of composite gels occurred by electro-osmosis and electrophoresis. Swelling and shrinking of the composite gels were controlled by the external stimuli of pH, voltage and the content of hydroxyapatite; therefore, it is proposed that composite gels would be good bone-filling material having the function of drug carriers.
Similar content being viewed by others
Introduction
Stimuli-responsive gels are known to change their shape, solubility and degree of ionization because of physical and chemical external stimuli.1, 2, 3 Physical stimuli include temperature, electric field, solvent, light, pressure, sound and magnetic field.4 Chemical or biochemical stimuli include pH, ions, and so on.4 Ionic gels are stimuli-responsive gels that change volume because of pH or electrical stimulus. These properties are used for a variety of devices such as drug carriers, artificial muscles and actuators.
Alginate gel, an ionic gel,5, 6, 7 has been widely used in drug carriers and tissue engineering.8, 9 Alginates are polysaccharides extracted from seaweed and electrolytes that have carboxyl groups. They are composed of two monomers, β-D-mannuronate and α-L-guluronate,10, 11 which are known to form gels in the presence of bivalent cations such as Ca2+.10, 11, 12
Hydroxyapatite is generated from calcium chloride (CaCl2) and diammonium hydrogenphosphate ((NH4)2HPO4).13 Hydroxyapatite, which has a good biocompatibility and a high biological affinity, is used as bone-filling material for treating bone defects caused by disease or injury. This material promotes the reproduction of new bone by injection into bone cavities. Even though hydroxyapatite has been used as a bone-filling material, it requires a long time to be replaced with host bone after implantation.14, 15, 16 Composite materials composed of hydroxyapatite and hydrogels would be suitable to fill large bone defects and would be easily absorbed by the patient’s body.
Composite gels were prepared from hydroxyapatite and calcium alginate gels. The preparation of both hydroxyapatite and calcium alginate uses CaCl2, and therefore hydroxyapatite/calcium alginate composite gels were prepared in one step. This composite gel could be applied as bone-filling material and would also have the function of a drug carrier.
Bone is a living tissue that is continually adapting to its biological environment through continuous formation and resorption.17 It is said that bone remodeling, a dynamic process, occurs in both cortical and trabecular (or cancellous) bone, allowing a rapid response to changes in circulating calcium levels.18, 19 Bone formation occurs when calcium is incorporated into the organic bone matrix secreted from osteoblasts.20 Bone resorption occurs when the organic bone matrix is decomposed by enzymes and by H+ ions from osteoclasts.20 In particular, the pH of resorbed lacunae after bone resorption decreases to 3–4.21
In this study, the stimuli-responsive characteristics of hydroxyapatite/calcium alginate composite gels were evaluated. As described above, the pH is changed in in vivo bone during bone remodeling. Therefore, the volume changes with pH of the composite gels were determined to apply them as drug carriers. In addition, the pH change is related to the movement of ions. Consequently, voltages were applied to the composite gels to move ions.
Experimental procedure
Materials and reagents
Calcium chloride (FW=110. 98), (NH4)2HPO4 (FW=132.06), 0.01 M hydrochloric acid (N/100) (HCl, 0.04% HCl in water), 0.01 M sodium hydroxide solution (N/100) (NaOH, 0.04% NaOH in water), thymol blue (C27H30O5S) (FW=466.59), methyl red (C15H15N3O2) (FW= 269.3), bromothymol blue (C27H28Br2O5S) (FW=624.38) and phenolphthalein (C20H14O4) (FW=318.32) were obtained from Kanto Chemical (Tokyo, Japan) and used without further purification. Sodium alginate (300 cps) was obtained from Nacalai Tesque (Kyoto, Japan) and used without further purification.
Preparation of hydroxyapatite/calcium alginate composite gel
Drops of (NH4)2HPO4 (120 mM and 480 mM)–1% sodium alginate aqueous solution were added to CaCl2 (250 mM and 1000 mM) aqueous solution, which was immersed in CaCl2 aqueous solution until equilibrium. Then, we obtained two types of composite gel: 250 mM CaCl2–120 mM (NH4)2HPO4–1% calcium alginate composite gels and 1000 mM CaCl2–480 mM (NH4)2HPO4−1% calcium alginate composite gels. In addition, calcium alginate gels were prepared from 1% sodium alginate aqueous solution and 100 mM CaCl2 aqueous solution. All the gels were immersed in distilled water, which was changed three times. The content of hydroxyapatite in the composite gels was measured with a thermogravimetrical analyzer.
Observation of pH dependence of composite gels
Composite gels and calcium alginate gels were immersed in distilled water. The gels were observed at 30-min intervals using a stereomicroscope (Shimadzu Manufacturing, Kyoto, Japan, STZ-168-BL/TL). The volume alteration ratio was calculated from the diameters of the composite gel and calcium alginate gel. The experiment was carried out with three types of gels (1% calcium alginate, 250 mM CaCl2–120 mM (NH4)2HPO4–1% calcium alginate and 1000 mM CaCl2–480 mM (NH4)2HPO4–1% calcium alginate) and using 0.01 M HCl or 0.01 M NaOH solutions instead of distilled water.
Observation of the electrochemical properties of composite gels
Various voltages were applied to the composite gels with a Pt mesh anode and a Pt rod cathode on the bottle of distilled water or 100 mM CaCl2 solution. Then, the gels were observed at 1-min intervals using a stereomicroscope (Shimadzu Manufacturing, STZ-168-BL/TL). The volume alteration ratio was calculated from the diameters of the composite gel and calcium alginate gel.
Observation of the pH dependence of the composite gels by electrical stimulus
Universal pH indicator solution was prepared by combining four types of dyes.22, 23 Thymol blue 0.005 g, methyl red 0.0125 g, bromothymol blue 0.05 g and phenolphthalein 0.10 g were dissolved in 100 ml of ethanol, neutralized with 0.1 M NaOH and diluted to a total volume of 200 ml with distilled water.
Composite gels and calcium alginate gels were immersed in the pH indicator solution for 1 day. Then, a voltage was applied to the composite gels.
Results and discussion
Preparation of hydroxyapatite/calcium alginate composite gel
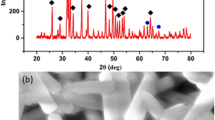
The composite gels were prepared with CaCl2 solution and (NH4)2HPO4–sodium alginate solution. The composite gels were white in color and spherical in shape. Figure 1 shows cross-sections of 1% alginate gel and 1000 mM CaCl2–480 mM (NH4)2HPO4 1% alginate gel. The cross-section of the 1% alginate gel was clear. In case of 1000 mM CaCl2–480 mM (NH4)2HPO4 1% alginate gel, the center of the cross-section was clear, and the exterior of the cross-section was white. It is considered that hydroxyapatite crystallizes at the exterior of the composite gels.
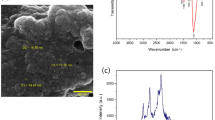
The crystallization of white hydroxyapatite in the composite gel was confirmed by X-ray diffraction and infrared spectroscopy. As a result of X-ray diffraction, the hydroxyapatite phases in the composite gels were confirmed from the peaks at 2θ=25.9° (002), 31.8° (211), 32.2° (112) and 32.9° (300).24 According to the results of infrared spectroscopy, absorption between 1100 and 1000 cm−1 corresponding to PO43− or HPO42− in the composite gels was observed. These results indicated that hydroxyapatite was successfully grown with CaCl2 and (NH4)2HPO4 in calcium alginate gel. The content of hydroxyapatite in the composite gel was measured with thermogravimetrical analyzer, and 49.9 and 61.8% hydroxyapatite was contained in the composite gel prepared from 250 mM CaCl2 and 120 mM (NH4)2HPO4, and 1000 mM CaCl2 and 480 mM (NH4)2HPO4, respectively.25 The content of hydroxyapatite in the gel was significantly related to the concentration and ratio of CaCl2 and (NH4)2HPO4.
Observation of the pH dependence of the composite gels
Stimulus response by pH was determined by immersing the composite gels in solutions of distilled water, 0.01 M HCl (pH 2) and 0.01 M NaOH (pH 10) (Figure 2). The gel volume was not changed by water immersion. However, the volume of the gel decreased after immersion in pH 2 solution. In contrast, the volume of the gel increased in pH 10 solution.
Figure 3 shows the volume alteration ratio of composite gels with pH over time. The volume of the gels before immersion into solutions of pH 2 and 10 was defined as 100%, and the volume alteration ratio of composite gels was calculated. The alginate gel and the hydroxyapatite/calcium alginate gel decreased in volume to about 30% after 3 h in an acidic solution of pH 2. The gels containing more hydroxyapatite decreased in volume more slowly than the gels containing less hydroxyapatite. All gels increased in volume in the alkaline solution of pH 10. Calcium alginate gels were 500% larger than the gels before immersion into the alkaline solution. The gels containing more hydroxyapatite showed little change in the alkaline solution. The gel became difficult to stretch after hydroxyapatite was generated.
Figure 4 shows the pH dependence of the 1000 mM CaCl2–480 mM (NH4)2HPO4 1% alginate gel after immersion for 2 h. The volume of the composite gel was constant between pH 5 and 10. The volume of the composite gel decreased below pH 3.
The change in shape of the gels attributed to pH was interpreted as follows (Figure 5).
In acidic solution, COO− ions and H+ ions bonded in the presence of a large quantity of H+ ions, and then the gel contracted because of the neutralized electrostatic repulsion.26, 27 In the alkaline solution, COO− ions and H+ ions were dissociated, and then the gel swelled because of H+ ion diffusion and electrostatic repulsion.25, 26 The shape change of the gel was caused by the electrostatic repulsion between carboxylate anions in the hydrogels.
Furthermore, the shrinkage and swelling of the gel was inhibited by the presence of hydroxyapatite. The solubility of hydroxyapatite is also pH responsive. Hydroxyapatite is soluble at lower pH and insoluble at higher pH.28, 29 It is suggested that the composite gel containing hydroxyapatite decreased in size as much as the alginate gel in HCl solution did, and the composite gel did not increase much in size in the NaOH solution. In the process of absorption of hydroxyapatite in vivo, hydroxyapatite must be dissolved. It is estimated that this composite gel is functional with high resolvability and high absorbance in vivo.
Observation of the electrochemical properties of the composite gels
Figure 6 shows photographs of the composite gels before and after applying voltage for an hour. In distilled water, the calcium alginate gels near the cathode increased in volume with applied voltage. Conversely, the hydroxyapatite/calcium alginate composite gels decreased in size with applied voltage. The calcium alginate gels became larger and the composite gels became smaller with a high applied voltage.
The shape change of the gels attributed to applied voltage was interpreted as follows (Figure 7). In the case of calcium alginate gels, the Ca2+ ions, which were used for cross-linking, and H3O+ ions, which were generated in the alginate gels, moved toward the cathode,26, 27, 30 and the water from the outside entered into the alginate gels. Therefore, the calcium alginate gel near the anode decreased in size, and the calcium alginate gel near the cathode increased in size. Figure 8 shows a side view of the 250 mM CaCl2–120 mM (NH4)2HPO4 1% alginate gel after applying voltage. The top of the gel was in contact with the cathode, and the bottom of the gel was in contact with the anode. In the case of composite gels, there is a large amount of hydroxyapatite. Ca2+ ions, which were part of the hydroxyapatite, also moved toward the cathode. In addition, H3O+ ions were generated in the composite gels. Therefore, the composite gels decreased in size because of the movement of H3O+ ions. The shrinkage of the hydrogels under applied voltage was due to electro-osmosis and electrophoresis.26, 27, 30
In the case of using the 100 mM CaCl2 solution instead of distilled water, Figure 9 shows the volume alteration ratio of the composite gels with electrical stimulus over time. The volume of the gels before applying the voltage was defined as 100%, and the volume alteration ratio of composite gels after applying the voltage was calculated. All of the gels decreased in size with voltage over time. The calcium alginate gels decreased by about 5% at 5 V after 5 min. The variation of the composite gels was less than that of the calcium alginate gels. The composite gels that contained a large amount of hydroxyapatite decreased in size less than the gels that contained a small amount of hydroxyapatite.
Observation of the pH dependence of the composite gels due to electrical stimulus
The pH indicator solution produced was dark green and changed color in solutions of various pH. The color was red at pH 4, orange at pH 5, yellow at pH 6, green at pH 7, blue at pH 8, indigo blue at pH 9 and violet at pH 10.
The composite gels and calcium alginate gels were immersed in a pH indicator solution. The composite gels were red, and the calcium alginate gels were green in the pH indicator solution. The color of all gels containing the pH indicator solution changed with the applied voltage (Figure 10).
The calcium alginate gels began to change from green to purple within a 2-min period. The majority of the gel changed to purple within 6 min. Conversely, the composite gels began to change from red to purple within 2 min, and the purple-colored area expanded with applied voltage over time. The gel containing a large amount of hydroxyapatite changed color more slowly than did the gels without hydroxyapatite. The content of hydroxyapatite was related to the color change of the gels. It is considered that the amount of charged electrolyte in the composite gels decreased with the amount of hydroxyapatite because ionic bonds were formed between the calcium and polyelectrolyte. As a result, the ions were not readily transported within the composite gels containing large amounts of hydroxyapatite.
In addition, the portion of gels in contact with the anode indicated alkalinity by turning a purple color. This occurred by removing the H3O+ in the gels attributed to the applied voltage. It is assumed that the stretching behavior was related to the movement of ions; therefore, the pH of the gels changed because of the applied voltage.
Conclusion
A hydroxyapatite/calcium alginate composite gel was synthesized in one step from (NH4)2HPO4–sodium alginate solution and CaCl2 solution. The change in volume of the composite gels was related to the pH and applied voltage. In the case of pH, the change in volume of the composite gels and calcium alginate gels was dependent on the dissociation of carboxylate anions with the environmental solution. In the case of electrical stimulus, the change in volume of the composite gels and calcium alginate gels depended on the movement of dissociated carboxylate anions because of the applied voltage. In addition, the stretching behavior of the composite gels depended on the content of hydroxyapatite. These composite gels were shown to have good bone-filling and drug release properties.
References
Kajiwara, K. & Ross-Murphy, S. B. Synthetic gels on the move. Nature 355, 208 (1992).
Osada, Y., Okuzaki, H. & Hori, H. A polymer gel with electrically driven motility. Nature 355, 242 (1992).
Gong, J. P., Nitta, T. & Osada, Y. Electrokinetic modeling of the contractile phenomena of polyelectrolyte gels. One-dimensional capillary model. J. Phys. Chem. 98, 9583 (1994).
El-Mohdy, H. L. A. & Safrany, A. Preparation of fast response superabsorbent hydrogels by radiation polymerization and crosslinking of N-isopropylacrylamide in solution. Rad. Phys. Chem. 77, 273 (2008).
Dentini, M., Rinaldi, G., Barbetta, A., Risica, D., Anselmi, C. & Skjåk-Bræk, G. Ionic gel formation of a (pseudo)alginate characterised by an alternating MG sequence produced by epimerising mannuronan with AlgE4. Carbohydrate Polym. 67, 465 (2007).
Hoad, C., Rayment, P., Cox, E., Wright, P., Butler, M., Spiller, R. & Gowland, P. Investigation of alginate beads for gastro-intestinal functionality, Part 2: In vivo characterization. Food Hydrocolloids 23, 833 (2009).
Skjåk-Bræk, G., Grasdalen, H. & Smidsrød, O. Inhomogeneous polysaccharide ionic gels. Carbohydrate Polym. 10, 31 (1989).
Lee, M., Lo, A. C., Cheung, P. T., Wong, D. & Chan, B. P. Drug carrier systems based on collagen–alginate composite structures for improving the performance of GDNF-secreting HEK293 cells. Biomaterials 30, 1214 (2009).
de Martimprey, H., Vauthier, C., Malvy, C. & Couvreur, P. Polymer nanocarriers for the delivery of small fragments of nucleic acids: oligonucleotides and siRNA. Eur. J. Pharm. Biopharm. 71, 490 (2009).
Straatmann, A. & Borchard, W. Phase separation in calcium alginate gels. Eur. Biophys. J. 32, 412 (2003).
Decho, A. W. Imaging an alginate polymer gel matrix using atomic force microscopy. Carbohydrate Res. 315, 330 (1999).
Liu, X. D., Yu, W. Y., Zhang, Y., Xue, W. M., Yu, W. T., Xiong, Y., Ma, X. J., Chen, Y. & Yuan, Q. Characterization of structure and diffusion behaviour of Ca-alginate beads prepared with external or internal calcium sources. J. Microencapsulation 19, 775 (2002).
Iwatsubo, T., Sumaru, K., Kanamori, T., Shinbo, T. & Yamaguchi, T. Construction of a new artificial biomineralization system. Biomacromolecules 7, 95 (2006).
Holmes, R. E., Bucholz, R. W. & Mooney, V. Porous hydroxyapatite as a bone-graft substitute in metaphyseal defects. A histometric study. J. Bone Joint Surg. 68A, 904 (1986).
Ishihara, K., Arai, J., Nakabayashi, N., Morita, S. & Furuya, K. Adhesive bone cement containing hydroxyapatite particle as bone compatible filler. J. Biomed. Mater. Res. 26, 937 (1992).
Tamai, N., Myoui, A., Tomita, T., Nakase, T., Tanaka, J., Ochi, T. & Yoshikawa, H. Novel hydroxyapatite ceramics with an interconnective porous structure exhibit superior osteoconduction in vivo. J. Biome. Mater. Res. 59, 110 (2002).
Penninger, C. L., Patel, N. M., Niebur, G. L., Tovar, A. & Renaud, J. E. A fully anisotropic hierarchical hybrid cellular automaton algorithm to simulate bone remodeling. Mech. Res. Comm. 35, 32 (2008).
Sims, N. A. & Gooi, J. H. Bone remodeling: multiple cellular interactions required for coupling of bone formation and resorption. Semin. Cell. Dev. Biol. 19, 444 (2008).
Pivonka, P., Zimak, J., Smith, D. W., Gardiner, B. S., Dunstan, C. R., Sims, N. A., Martin, T.J. & Mundy, G.R. Model structure and control of bone remodeling: a theoretical study. Bone 43, 249 (2008).
Sakai, H. & Kuno, M. Ion signaling mechanisms in bone remodeling. Igaku no Ayumi 199, 199 (2001).
Ozawa, H., Ejiri, S. & Nakamura, H. Cell biological aspects on bone resorption. Tanp. Kaku. Koso. 40, 492 (1995).
Yamada, S. Indicator for concentration of hydrogen ion. Chem. Abstr. 28, 2258 (1934)Japanese Patent 99, 664, 21 February (1993).
Laurence, S. F. & Gruntfest, I. J. Demonstration experiments using universal indicators. J. Chem. Educ. 14, 274 (1937).
Taguchi, T., Kishida, A. & Akashi, M. Hydroxyapatite formation on/in poly(vinyl alcohol) hydrogel matrices using a novel alternate soaking process. Chem. Lett. 27, 711 (1998).
Obara, S., Saito, H., Yamauchi, T. & Tsubokawa, N. Preparation of hydroxyapatite/calcium alginate composite gel and application to medical material In: SAMPE 2008, Technical Conference Proceedings: Material and Process Innovations: Changing our World; 18–22 May 2008; Long Beach, CA, USA. Society for the Advancement of Material and Process Engineering, Covina, CA, USA; CD-ROM-7pp.
Qiu, Y. & Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 53, 321 (2001).
Gutowska, A., Bark, J.S., Kwon, I. C., Bae, U. H., Cha, Y. & Kim, S. W. Squeezing hydrogels for controlled oral drug delivery. J. Controlled Release 48, 141 (1997).
Okazaki, M. ‘Chemistry of Apatites’, Tokai University, Kanagawa, Japan (1992).
Nonami, T., Funakoshi, K., Hase, H. & Tone, K. Application of apatite cated titanium dioxide. J. Surface Finish. Soc. Jpn. 55, 335 (2004).
Sawahata, K., Hara, M., Yasunaga, H. & Osada, Y. Electrically controlled drug delivery system using polyelectrolyte gels. J. Controlled Release 14, 253 (1990).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Obara, S., Yamauchi, T. & Tsubokawa, N. Evaluation of the stimulus response of hydroxyapatite/ calcium alginate composite gels. Polym J 42, 161–166 (2010). https://doi.org/10.1038/pj.2009.310
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2009.310
Keywords
This article is cited by
-
Gelation Modification of Alginate Nonwoven Fabrics
Fibers and Polymers (2018)
-
Hydroxyapatite formation on oxidized cellulose nanofibers in a solution mimicking body fluid
Polymer Journal (2015)
-
CaCO3/chitin-whisker hybrids: formation of CaCO3 crystals in chitin-based liquid-crystalline suspension
Polymer Journal (2010)