Abstract
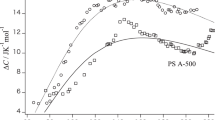
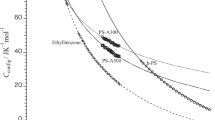
A study was carried out on the effects of thermal aging on specific volume V and specific enthalpy H of polystyrene below the glass transition temperature. The dependence of V and H on annealing time was similar. No significant effect of the distribution of molecular weight on the relaxation behavior was found. The behavior of V and H reaching a maximum after a double-step temperature-jump was also investigated. The time for the maximum V and H varied in proportion to the period of pre-annealing. The time for the maximum volume was slightly longer than that for the maximum enthalpy. These facts indicate that the dependence of V and H on the order parameters specifying the glassy state are similar but not exactly the same. From the enthalpy and volume relaxation curves, the enthalpy required for creating free volumes in polystyrene was calculated to be 2.0 kJ cm−3. The behavior of the volume relaxation was discussed by assuming a wide distribution of relaxation time.
Similar content being viewed by others
Article PDF
References
S. E. B. Petrie, “Polymeric Materials: Relationships between Structure and Mechanical Behavior,” American Society for Metals, Metal Park, Ohio, 1975, pp 55—118.
M. R. Tant and G. L. Wilkes, Polym. Eng. Sci., 21, 874 (1981).
A. Q. Tool, J. Res. Natl. Bur. Stand., 37, 73 (1946).
T. G Fox and P. J. Flory, J. Appl. Phys., 40, 4254 (1969).
A. J. Kovacs, J. Polym. Sci., 30, 131 (1958).
A. J. Kovacs, Fortschr. Hochpolym. Forsch., 3, 394 (1963).
M. Goldstein and M. Nakonecznyj, Phys. Chem. Glasses, 6, 126 (1965).
H. Endo, T. Fujimoto, and M. Nagasawa, J. Polym. Sci., A-2, 7, 1669 (1970).
S. Hozumi, Polym. J., 2, 756 (1971).
M. Uchidoi, K. Adachi, and Y. Ishida, Polym. J., 10, 161 (1978).
S. E. B. Petrie, J. Polym. Sci., A-2, 10, 1255 (1972).
R. Straff and D. R. Uhlmann, J. Polym. Sci., Polym. Chem. Ed., 14, 1087 (1976).
H. E. Bair, G. E. Johnson, E. W. Anderson, and S. Matsuoka, Polym. Eng. Sci., 21, 930 (1981).
R. O. Davies and G. O. Jones, Proc. R. Soc. London Ser. A, 217, 26 (1953).
G. Rehage, J. Macromol. Sci., Phys., B18, 423 (1980).
P. K. Gupta and C. T. Moynihan, J. Chem. Phys., 65, 4136 (1976).
R.-J. Roe, J. Appl. Phys., 48, 4085 (1977).
R. M. Kimmel and D. R. Uhlmann, J. Appl. Phys., 40, 4254 (1969).
L. C. E. Struik, Rheol. Acta, 5, 303 (1966).
S. Matsuoka, Polym. Eng. Sci., 21, 907 (1981).
A. K. Doolittle, J. Appl. Phys., 22, 1471 (1951).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Adachi, K., Kotaka, T. Volume and Enthalpy Relaxation in Polystyrene. Polym J 14, 959–970 (1982). https://doi.org/10.1295/polymj.14.959
Issue Date:
DOI: https://doi.org/10.1295/polymj.14.959
Keywords
This article is cited by
-
Modeling the density relaxation of polystyrene
Rheologica Acta (2022)
-
Determination of the glass transition temperature
Journal of Thermal Analysis and Calorimetry (2009)
-
Structural Relaxation of Acetaminophen Glass
Pharmaceutical Research (2006)
-
Aging bulk modulus obtained from enthalpy and volume relaxations of a-PMMA and its blends with PEO
Mechanics of Time-Dependent Materials (2006)
-
Pulsed dielectric spectroscopy of supercooled liquids
Zeitschrift für Physik B Condensed Matter (1995)