Abstract
Background:
Surgery and radiation-based therapies are standard management options for men with clinically localized high-risk prostate cancer (PCa). Contemporary patterns of care are unknown. We hypothesize the use of surgery has steadily increased in more recent years.
Methods:
Using the National Cancer Data Base for 2004–2013, all men diagnosed with high-risk localized PCa were identified using National Comprehensive Cancer Network criteria. Temporal trends in initial management were assessed. Multivariable logistic regression was used to evaluate demographic and clinical factors associated with undergoing radical prostatectomy (RP).
Results:
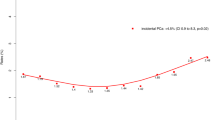
In total, 127 391 men were identified. Use of RP increased from 26% in 2004 to 42% in 2013 (adjusted risk ratio (RR) 1.51, 95% CI 1.42–1.60, P<0.001), while external beam radiation therapy (EBRT) decreased from 49% to 42% (P<0.001). African American men had lower odds of undergoing RP (unadjusted rate of 28%, adjusted RR 0.69, 95% CI 0.66–0.72, <0.001) compared to White men (37%). Age was inversely associated with likelihood of receiving RP. Having private insurance was significantly associated with the increased use of RP (vs Medicare, adjusted odds ratio 1.04, 95% CI 1.01–1.08, P=0.015). Biopsy Gleason scores 8–10 with and without any primary Gleason 5 pattern were associated with decreased odds of RP (vs Gleason score ⩽6, both P<0.001). Academic and comprehensive cancer centers were more likely to perform RP compared to community hospitals (both P<0.001).
Conclusion:
The likelihood of receiving RP for high-risk PCa dramatically increased from 2004 to 2013. By 2013, the use of RP and EBRT were similar. African American men, elderly men and those without private insurance were less likely to receive RP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A . Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30.
Mohler JL, Armstrong AJ, Bahnson RR, D'Amico AV, Davis BJ, Eastham JA et al. Prostate cancer, version 1, 2016. J Natl Compr Canc Netw 2016; 14: 19–30.
Moyer VA, U.S. Preventive Services Task Force.. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 157: 120–134.
Drazer MW, Huo D, Eggener SE . National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate-specific antigen-based screening. J Clin Oncol 2015; 33: 2416–2423.
Barocas DA, Mallin K, Graves AJ, Penson DF, Palis B, Winchester DP et al. Effect of the USPSTF Grade D recommendation against screening for prostate cancer on incident prostate cancer diagnoses in the United States. J Urol 2015; 194: 1587–1593.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 2014; 65: 124–137.
Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 2012; 367: 203–213.
Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS et al. Fifteen-year survival outcomes following primary androgen-deprivation therapy for localized prostate cancer. JAMA Intern Med 2014; 174: 1460–1467.
Warde P, Mason M, Ding K, Kirkbride P, Brundage M, Cowan R et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet 2011; 378: 2104–2111.
Cooperberg MR, Vickers AJ, Broering JM, Carroll PR . Comparative risk-adjusted mortality outcomes after primary surgery, radiotherapy, or androgen-deprivation therapy for localized prostate cancer. Cancer 2010; 116: 5226–5234.
Zelefsky MJ, Eastham JA, Cronin AM, Fuks Z, Zhang Z, Yamada Y et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol 2010; 28: 1508–1513.
Petrelli F, Vavassori I, Coinu A, Borgonovo K, Sarti E, Barni S . Radical prostatectomy or radiotherapy in high-risk prostate cancer: a systematic review and metaanalysis. Clin Genitourin Cancer 2014; 12: 215–224.
Wallis CJ, Saskin R, Choo R, Herschorn S, Kodama RT, Satkunasivam R et al. Surgery versus radiotherapy for clinically-localized prostate cancer: a systematic review and meta-analysis. Eur Urol 2016; 70: 21–30.
Winchester DP, Stewart AK, Bura C, Jones RS . The National Cancer Data Base: a clinical surveillance and quality improvement tool. J Surg Oncol 2004; 85: 1–3.
Schymura MJ, Sun L, Percy-Laurry A . Prostate cancer collaborative stage data items—their definitions, quality, usage, and clinical implications: a review of SEER data for 2004-2010. Cancer 2014; 120: 3758–3770.
Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014; 370: 932–942.
Berglund A, Garmo H, Tishelman C, Holmberg L, Stattin P, Lambe M . Comorbidity, treatment and mortality: a population based cohort study of prostate cancer in PCBaSe Sweden. J Urol 2011; 185: 833–839.
American College of Surgeons. Cancer Program Categories. Available at http://www.facs.org/quality-programs/cancer/coc/apply/categories. Accessed on 25 March 2016..
Zou G . A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159: 702–706.
Panageas KS, Schrag D, Riedel E, Bach PB, Begg CB . The effect of clustering of outcomes on the association of procedure volume and surgical outcomes. Ann Intern Med 2003; 139: 658–665.
Boorjian SA, Karnes RJ, Viterbo R, Rangel LJ, Bergstralh EJ, Horwitz EM et al. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer 2011; 117: 2883–2891.
Pierorazio PM, Guzzo TJ, Han M, Bivalacqua TJ, Epstein JI, Schaeffer EM et al. Long-term survival after radical prostatectomy for men with high Gleason sum in pathologic specimen. Urology 2010; 76: 715–721.
Lu-Yao GL, Yao SL . Population-based study of long-term survival in patients with clinically localised prostate cancer. Lancet 1997; 349: 906–910.
Barry MJ, Albertsen PC, Bagshaw MA, Blute ML, Cox R, Middleton RG et al. Outcomes for men with clinically nonmetastatic prostate carcinoma managed with radical prostactectomy, external beam radiotherapy, or expectant management: a retrospective analysis. Cancer 2001; 91: 2302–2314.
Zeliadt SB, Ramsey SD, Penson DF, Hall IJ, Ekwueme DU, Stroud L et al. Why do men choose one treatment over another?: a review of patient decision making for localized prostate cancer. Cancer 2006; 106: 1865–1874.
Tewari A, Divine G, Chang P, Shemtov MM, Milowsky M, Nanus D et al. Long-term survival in men with high grade prostate cancer: a comparison between conservative treatment, radiation therapy and radical prostatectomy—a propensity scoring approach. J Urol 2007; 177: 911–915.
Merglen A, Schmidlin F, Fioretta G, Verkooijen HM, Rapiti E, Zanetti R et al. Short- and long-term mortality with localized prostate cancer. Arch Intern Med 2007; 167: 1944–1950.
Albertsen PC, Hanley JA, Penson DF, Barrows G, Fine J . 13-year outcomes following treatment for clinically localized prostate cancer in a population based cohort. J Urol 2007; 177: 932–936.
Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375: 1415–1424.
Cooperberg MR, Carroll PR . Trends in management for patients with localized prostate cancer, 1990-2013. JAMA 2015; 314: 80–82.
Martin JM, Handorf EA, Kutikov A, Uzzo RG, Bekelman JE, Horwitz EM et al. The rise and fall of prostate brachytherapy: use of brachytherapy for the treatment of localized prostate cancer in the National Cancer Data Base. Cancer 2014; 120: 2114–2121.
Morris W, Tyldesley S, Rodda S, Halperin R, Pai H, McKenzie M et al. LDR brachytherapy is superior to 78 Gy of EBRT for unfavourable risk prostate cancer: the results of a randomized trial. Radiother Oncol 2015; 115: S239.
Mason MD, Parulekar WR, Sydes MR, Brundage M, Kirkbride P, Gospodarowicz M et al. Final report of the intergroup randomized study of combined androgen-deprivation therapy plus radiotherapy versus androgen-deprivation therapy alone in locally advanced prostate cancer. J Clin Oncol 2015; 33: 2143–2150.
Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 2009; 373: 301–308.
Moses KA, Orom H, Brasel A, Gaddy J, Underwood W 3rd . Racial/ethnic disparity in treatment for prostate cancer: does cancer severity matter. Urology 2016; 99: 76–83.
Schwartz K, Powell IJ, Underwood W 3rd, George J, Yee C, Banerjee M . Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology 2009; 74: 1296–1302.
Fossati N, Nguyen DP, Trinh QD, Sammon J, Sood A, Larcher A et al. The impact of insurance status on tumor characteristics and treatment selection in contemporary patients with prostate cancer. J Natl Compr Canc Netw 2015; 13: 1351–1358.
Roetzheim RG, Pal N, Gonzalez EC, Ferrante JM, Van Durme DJ, Krischer JP . outcomes. Am J Public Health 2000; 90: 1746–1754.
Warren JL, Butler EN, Stevens J, Lathan CS, Noone AM, Ward KC et al. Receipt of chemotherapy among medicare patients with cancer by type of supplemental insurance. J Clin Oncol 2015; 33: 312–318.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
A poster version of these data was presented at the 16th Annual Meeting of the Society of Urologic Oncology in Washington, DC, December 2015.
Supplementary Information accompanies the paper on the Prostate Cancer and Prostatic Diseases website
Rights and permissions
About this article
Cite this article
Weiner, A., Matulewicz, R., Schaeffer, E. et al. Contemporary management of men with high-risk localized prostate cancer in the United States. Prostate Cancer Prostatic Dis 20, 283–288 (2017). https://doi.org/10.1038/pcan.2017.5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2017.5
This article is cited by
-
Surgical Management and Considerations for Patients with Localized High-Risk Prostate Cancer
Current Treatment Options in Oncology (2024)
-
Comparison of the treatment of men with prostate cancer between the US and England: an international population-based study
Prostate Cancer and Prostatic Diseases (2023)
-
Quantifying treatment selection bias effect on survival in comparative effectiveness research: findings from low-risk prostate cancer patients
Prostate Cancer and Prostatic Diseases (2021)
-
Surgical management of high-risk, localized prostate cancer
Nature Reviews Urology (2020)
-
Plasma metabolomic profile in prostatic intraepithelial neoplasia and prostate cancer and associations with the prostate-specific antigen and the Gleason score
Metabolomics (2020)