Abstract
Background:
Several randomized controlled trials assessed the outcomes of patients treated with neoadjuvant hormonal therapy (NHT) before radical prostatectomy (RP). The majority of them included mainly low and intermediate risk prostate cancer (PCa) without specifically assessing PCa-related death (PCRD). Thus, there is a lack of knowledge regarding a possible effect of NHT on PCRD in the high-risk PCa population. We aimed to analyze the effect of NHT on PCRD in a multicenter high-risk PCa population treated with RP, using a propensity-score adjustment.
Methods:
This is a retrospective multi-institutional study including patients with high-risk PCa defined as: clinical stage T3–4, PSA >20 ng ml−1 or biopsy Gleason score 8–10. We compared PCRD between RP and NHT+RP using competing risks analysis. Correction for group differences was performed by propensity-score adjustment.
Results:
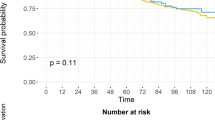
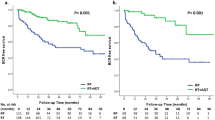
After application of the inclusion/exclusion criteria, 1573 patients remained for analysis; 1170 patients received RP and 403 NHT+RP. Median follow-up was 56 months (interquartile range 29–88). Eighty-six patients died of PCa and 106 of other causes. NHT decreased the risk of PCRD (hazard ratio (HR) 0.5; 95% confidence interval (CI) 0.32–0.80; P=0.0014). An interaction effect between NHT and radiotherapy (RT) was observed (HR 0.3; 95% CI 0.21–0.43; P<0.0008). More specifically, of patients who received adjuvant RT, those who underwent NHT+RP had decreased PCRD rates (2.3% at 5 year) compared to RP (7.5% at 5 year). The retrospective design and lack of specific information about NHT are possible limitations.
Conclusions:
In this propensity-score adjusted analysis from a large high-risk PCa population, NHT before surgery significantly decreased PCRD. This effect appeared to be mainly driven by the early addition of RT post-surgery. The specific sequence of NHT+RP and adjuvant RT merits further study in the high-risk PCa population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol 2017; 71: 618–629.
Mohler JL, Kantoff PW, Armstrong AJ, Bahnson RR, Cohen M, D'Amico AV et al. Prostate cancer, version 2.2014. J Natl Compr Canc Netw 2014; 12: 686–718.
Kumar S, Shelley M, Harrison C, Coles B, Wilt TJ, Mason MD . Neo-adjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer. Cochrane Database Syst Rev. 2006; 4: CD006019.
Bartek J, Mistrik M, Bartkova J . Androgen receptor signaling fuels DNA repair and radioresistance in prostate cancer. Cancer Discov 2013; 3: 1222–1224.
Al-Ubaidi FL, Schultz N, Loseva O, Egevad L, Granfors T, Helleday T . Castration therapy results in decreased Ku70 levels in prostate cancer. Clin Cancer Res. 2013; 19: 1547–1556.
Joniau S, Van den Bergh L, Lerut E, Deroose CM, Haustermans K, Oyen R et al. Mapping of pelvic lymph node metastases in prostate cancer. Eur Urol. 2013; 63: 450–458.
Fine J, Gray R . A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
Rosenbaum PR, Rubin DB . The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70: 41–55.
Lin DY, Wei LJ . The Robust Inference for the Cox Proportional Hazards Model. J Am Stat Assoc 1989; 84: 1074–1078.
Abdollah F, Sun M, Thuret R, Jeldres C, Tian Z, Briganti A et al. Lymph node count threshold for optimal pelvic lymph node staging in prostate cancer. Int J Urol 2012; 19: 645–651.
Berglund RK, Tangen CM, Powell IJ, Lowe BA, Haas GP, Carroll PR et al. Ten-year follow-up of neoadjuvant therapy with goserelin acetate and flutamide before radical prostatectomy for clinical T3 and T4 prostate cancer: update on Southwest Oncology Group Study 9109. Urology 2012; 79: 633–637.
Moltzahn F, Karnes J, Gontero P, Kneitz B, Tombal B, Bader P et al. Predicting prostate cancer-specific outcome after radical prostatectomy among men with very high-risk cT3b/4 PCa: a multi-institutional outcome study of 266 patients. Prostate Cancer Prostatic Dis 2015; 18: 31–37.
Joniau S, Hsu CY, Gontero P, Spahn M, Van Poppel H . Radical prostatectomy in very high-risk localized prostate cancer: long-term outcomes and outcome predictors. Scand J Urol Nephrol 2012; 46: 164–171.
Johnstone PA, Ward KC, Goodman M, Assikis V, Petros JA . Radical prostatectomy for clinical T4 prostate cancer. Cancer 2006; 106: 2603–2609.
Culp SH, Schellhammer PF, Williams MB . Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol 2014; 65: 1058–1066.
Taplin ME, Montgomery B, Logothetis CJ, Bubley GJ, Richie JP, Dalkin BL et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J Clin Oncol 2014; 32: 3705–3715.
Montgomery B, Tretiakova MS, Joshua AM, Gleave ME, Fleshner N, Bubley GJ et al. Neoadjuvant enzalutamide prior to prostatectomy. Clin Cancer Res 2016 (doi:10.1158/1078-0432.CCR-16-1357).
Efstathiou E, Davis JW, Titus MA et al. Neoadjuvant enzalutamide (ENZA) and abiraterone acetate (AA) plus leuprolide acetate (LHRHa) versus AA+ LHRHa in localized high-risk prostate cancer (LHRPC). J Clin Oncol 2016; 34: (suppl; abstr 5002).
Jorgensen TJ . Enhancing radiosensitivity: targeting the DNA repair pathways. Cancer Biol Ther 2009; 8: 665–670.
Polkinghorn WR, Parker JS, Lee MX, Kass EM, Spratt DE, Iaquinta PJ et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov 2013; 3: 1245–1253.
Goodwin JF, Schiewer MJ, Dean JL, Schrecengost RS, de Leeuw R, Han S et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov 2013; 3: 1254–1271.
Tarish FL, Schultz N, Tanoglidi A, Hamberg H, Letocha H, Karaszi K et al. Castration radiosensitizes prostate cancer tissue by impairing DNA double-strand break repair. Sci Transl Med 2015; 7: 312re11.
Jackson WC, Schipper MJ, Johnson SB, Foster C, Li D, Sandler HM et al. Duration of androgen deprivation therapy influences outcomes for patients receiving radiation therapy following radical prostatectomy. Eur Urol 2016; 69: 50–57.
Acknowledgements
Funding for the costs of the statistical analyses was from the Leuven Cancer Institute, J. De Wever Fonds and Federico Foundation.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
LT: research grants from Bayer, Ipsen, Ferring, Janssen; consulting or advisory role for Ipsen; travel, accommodation, expenses from Astellas, Bayer and Pierre-Fabre. SJ: company consultant for Astellas, Ipsen, Bayer, Sanofi and Janssen; has received company speaker honoraria from Astellas, Amgen, Bayer, Sanofi, Janssen and Ipsen; has participated in trials for Astellas, Janssen and Bayer; has received fellowship and travel grants from Astellas, Amgen, Bayer, Sanofi, Janssen, Ipsen and Pfizer; and has received grant and research support from Astellas, Bayer and Janssen. MA: travel, accommodation, expenses from Astellas, Amgen and Bayer. WE: travel, accommodation, expenses from Astellas. RJK: research funding from GenomeDX; patents, royalties, other intellectual property from GenomeDX. PG: honoraria from Janssen, Ipsen; Speaker’s Bureau from Medacs; research funding from Astellas; travel, accommodation, expenses from Janssen. TVdB: travel, accommodation, expenses from Ipsen. FKC: consulting or advisory role from Astellas, UroTech. HVDP: honoraria from Intuitive Surgical; consulting or advisory role from Astellas; research funding from Astellas, Storz; travel, accommodation, expenses from Intuitive Surgical, Storz. BT: honoraria from Amgen, Astellas, Bayer, Ferring, Sanofi and Janssen; consulting or advisory role—Astellas, Bayer, Ferring, Janssen, Takeda, Steba Biotech, Sanofi; Speaker’s Bureau—Amgen, Janssen; research funding—Ferring; travel, accommodation, expenses—Amgen, Astellas, Bayer, Ferring Janssen, Sanofi. GM: Speaker’s Bureau from Ipsen, Takeda; travel, accommodation, expenses from Ipsen, Takeda and Ferring. GDM: honoraria from Astellas, Ipsen, AstraZeneca, Bayer, Ferring, Sanofi, Janssen; consulting or advisory role—Astellas, Bayer, Ferring, Janssen, Ipsen, Sanofi; research funding—Ipsen; travel, accommodation, expenses—all of the above. AB: honoraria from Astellas, Ipsen, Ferring; consulting or advisor role from Janssen; Speaker’s Bureau from Ipsen, Ferring and Janssen; travel, accommodation, expenses from Ipsen. Remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tosco, L., Laenen, A., Briganti, A. et al. The survival impact of neoadjuvant hormonal therapy before radical prostatectomy for treatment of high-risk prostate cancer. Prostate Cancer Prostatic Dis 20, 407–412 (2017). https://doi.org/10.1038/pcan.2017.29
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2017.29