Abstract
Background:
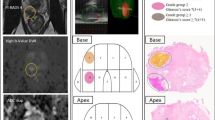
The objective of this study was to analyze the potential of prostate magnetic resonance imaging (MRI) and MRI/transrectal ultrasound-fusion biopsies to detect and to characterize significant prostate cancer (sPC) in the anterior fibromuscular stroma (AFMS) and in the transition zone (TZ) of the prostate and to assess the accuracy of multiparametric MRI (mpMRI) and biparametric MRI (bpMRI) (T2w and diffusion-weighted imaging (DWI)).
Methods:
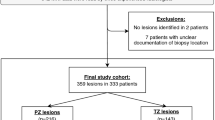
Seven hundred and fifty-five consecutive patients underwent prebiopsy 3 T mpMRI and transperineal biopsy between October 2012 and September 2014. MRI images were analyzed using PIRADS (Prostate Imaging-Reporting and Data System). All patients had systematic biopsies (SBs, median n=24) as reference test and targeted biopsies (TBs) with rigid software registration in case of MRI-suspicious lesions. Detection rates of SBs and TBs were assessed for all PC and sPC patients defined by Gleason score (GS)⩾3+4 and GS⩾4+3. For PC, which were not concordantly detected by TBs and SBs, prostatectomy specimens were assessed. We further compared bpMRI with mpMRI.
Results:
One hundred and ninety-one patients harbored 194 lesions in AFMS and TZ on mpMRI. Patient-based analysis detected no difference in the detection of all PC for SBs vs TBs in the overall cohort, but in the repeat-biopsy population TBs performed significantly better compared with SBs (P=0.004 for GS⩾3+4 and P=0.022 for GS⩾4+3, respectively). Nine GS⩾4+3 sPCs were overlooked by SBs, whereas TBs missed two sPC in men undergoing primary biopsy. The combination of SBs and TBs provided optimal local staging. Non-inferiority analysis showed no relevant difference of bpMRI to mpMRI in sPC detection.
Conclusions:
MRI-targeted biopsies detected significantly more anteriorly located sPC compared with SBs in the repeat-biopsy setting. The more cost-efficient bpMRI was statistically not inferior to mpMRI in sPC detection in TZ/AFMS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 2014; 65: 124–137.
Mygatt J, Sesterhenn I, Rosner I, Chen Y, Cullen J, Morris-Gore T et al. Anterior tumors of the prostate: clinicopathological features and outcomes. Prostate Cancer Prostat Dis 2014; 17: 75–80.
Nevoux P, Ouzzane A, Ahmed HU, Emberton M, Montironi R, Presti JC Jr et al. Quantitative tissue analyses of prostate cancer foci in an unselected cystoprostatectomy series. BJU Int 2012; 110: 517–523.
Ploussard G, Nicolaiew N, Marchand C, Terry S, Vacherot F, Vordos D et al. Prospective evaluation of an extended 21-core biopsy scheme as initial prostate cancer diagnostic strategy. Eur Urol 2014; 65: 154–161.
Radtke JP, Kuru TH, Boxler S, Alt CD, Popeneciu I V, Huettenbrink C et al. Comparative analysis of transperineal template saturation prostate biopsy versus magnetic resonance imaging targeted biopsy with magnetic resonance imaging-ultrasound fusion guidance. J Urol 2015; 193: 87–94.
Hoeks CM, Hambrock T, Yakar D, Hulsbergen-van de Kaa CA, Feuth T, Witjes JA et al. Transition zone prostate cancer: detection and localization with 3-T multiparametric MR imaging. Radiology 2013; 266: 207–217.
Ouzzane A, Puech P, Lemaitre L, Leroy X, Nevoux P, Betrouni N et al. Combined multiparametric MRI and targeted biopsies improve anterior prostate cancer detection, staging, and grading. Urology 2011; 78: 1356–1362.
Noguchi M, Stamey TA, Neal JE, Yemoto CE . An analysis of 148 consecutive transition zone cancers: clinical and histological characteristics. J Urol 2000; 163: 1751–1755.
Chun FK, Briganti A, Jeldres C, Erbersdobler A, Schlomm T, Steuber T et al. Zonal origin of localized prostate cancer does not affect the rate of biochemical recurrence after radical prostatectomy. Eur Urol 2007; 51: 949–955; discussion 955.
Pokorny MR, e Rooij M, Duncan E, Schröder FH, Parkinson R, Barentsz JO et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-fuided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol 2014; 66: 22–29.
Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313: 390.
Volkin D, Turkbey B, Hoang AN, Rais-Bahrami S, Yerram N, Walton-Diaz A et al. Multiparametric magnetic resonance imaging (MRI) and subsequent MRI/ultrasonography fusion-guided biopsy increase the detection of anteriorly located prostate cancers. BJU Int 2014; 114: E43–E49.
Le JD, Tan N, Shkolyar E, Lu DY, Kwan L, Marks LS et al. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Eur Urol 2015; 67: 569–576.
Baco E, Ukimura O, Rud E, Vlatkovic L, Svindland A, Aron M et al. Magnetic resonance imaging-iransectal ultrasound image-fusion biopsies accurately characterize the index tumor: correlation with step-sectioned radical prostatectomy specimens in 135 patients. Eur Urol 2015; 67: 787–794.
Chesnais AL, Niaf E, Bratan F, Mege-Lechevallier F, Roche S, Rabilloud M et al. Differentiation of transitional zone prostate cancer from benign hyperplasia nodules: evaluation of discriminant criteria at multiparametric MRI. Clin Radiol 2013; 68: e323–e330.
Bouyé S, Potiron E, Puech P, Leroy X, Lemaitre L, Villers A . Transition zone and anterior stromal prostate cancers: zone of origin and intraprostatic patterns of spread at histopathology. Prostate 2009; 69: 105–113.
McNeal JE . The zonal anatomy of the prostate. Prostate 1981; 2: 35–49.
Schimmöller L, Quentin M, Arsov C, Hiester A, Buchbender C, Rabenalt R et al. MR-sequences for prostate cancer diagnostics: validation based on the PI-RADS scoring system and targeted MR-guided in-bore biopsy. Eur Radiol 2014; 24: 2582–2589.
Siddiqui MM, Rais-Bahrami S, Truong H, Stamatakis L, Vourganti S, Nix J et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol 2013; 64: 713–719.
Rud E, Klotz D, Rennesund K, Baco E, Berge V, Lien D et al. Detection of the index tumor and tumor volume in prostate cancer using T2w and DW MRI alone. BJU Int 2014; 114: E32–E42.
Kuru TH, Roethke MC, Seidenader J, Simpfendorfer T, Boxler S, Alammar K et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol 2013; 190: 1380–1386.
Moore CM, Kasivisvanathan V, Eggener S, Emberton M, Fütterer JJ, Gill IS et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol 2013; 64: 544–552.
Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G et al. ESUR prostate MR guidelines 2012. Eur Radiol 2012 22: 746–757.
Radtke JP, Kuru TH, Boxler S, Alt CD, Popeneciu I V, Huettenbrink C et al. Comparative analysis of transperineal template saturation prostate biopsy versus magnetic resonance imaging targeted biopsy with magnetic resonance imaging-ultrasound fusion guidance. J Urol 2015; 193: 87–94.
Egevad L, Srigley JR, Delahunt B . International society of urological pathology consensus conference on handling and staging of radical prostatectomy specimens. Adv Anat Pathol 2011; 18: 301–305.
Schmid HP, McNeal JE . An abbreviated standard procedure for accurate tumor volume estimation in prostate cancer. Am J Surg Pathol 1992; 16: 184–191.
Komai Y, Numao N, Yoshida S, Matsuoka Y, Nakanishi Y, Ishii C et al. High diagnostic ability of multiparametric magnetic resonance imaging to detect anterior prostate cancer missed by transrectal 12-core biopsy. J Urol 2013; 190: 867–873.
Kasivisvanathan V, Dufour R, Moore CM, Ahmed HU, Abd-Alazeez M, Charman SC et al. Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol 2013; 189: 860–866.
Rais-Bahrami S, Siddiqui MM, Vourganti S, Turkbey B, Rastinehad AR, Stamatakis L et al. Diagnostic value of biparametric magnetic resonance imaging (MRI) as an adjunct to prostate-specific antigen (PSA)-based detection of prostate cancer in men without prior biopsies. BJU Int 2014; 115: 381–388.
Wolters T, Roobol MJ, van Leeuwen PJ, van den Bergh RC, Hoedemaeker RF, van Leenders GJ et al. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J Urol 2011; 185: 121–125.
American College of Radiology. MR Prostate Imaging Reporting and Data System version 2.0. Available at http://www.acr.org/Quality-Safety/Resources/PIRADS.
Rosenkrantz AB, Kim S, Campbell N, Gaing B, Deng F-M, Taneja SS . Transition zone prostate cancer: revisiting the role of multiparametric MRI at 3 T. Am J Roentgenol 2015; 204: W266–W272.
Delongchamps NB, Beuvon F, Eiss D, Flam T, Muradyan N, Zerbib M et al. Multiparametric MRI is helpful to predict tumor focality, stage, and size in patients diagnosed with unilateral low-risk prostate cancer. Prostate Cancer Prostatic Dis 2011; 14: 232–237.
Thompson JE, Moses D, Shnier R, Brenner P, Delprado W, Ponsky L et al. Multi-parametric magnetic resonance imaging guiding diagnostic biopsy detects significant prostate cancer, and could reduce unnecessary biopsies and over-detection: a prospective study. J Urol 2014; 192: 67–74.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
BAH is grateful for funding from the German Research Foundation, and the European Foundation for Urology. BAH has received research funding from MedCom and Uromed. None of these sources had any input whatsoever into this article. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Prostate Cancer and Prostatic Diseases website
Rights and permissions
About this article
Cite this article
Radtke, J., Boxler, S., Kuru, T. et al. Improved detection of anterior fibromuscular stroma and transition zone prostate cancer using biparametric and multiparametric MRI with MRI-targeted biopsy and MRI-US fusion guidance. Prostate Cancer Prostatic Dis 18, 288–296 (2015). https://doi.org/10.1038/pcan.2015.29
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2015.29
This article is cited by
-
Systematische oder gezielte Fusionsbiopsie der Prostata
Die Urologie (2023)
-
Concordance between biparametric MRI, transperineal targeted plus systematic MRI-ultrasound fusion prostate biopsy, and radical prostatectomy pathology
Scientific Reports (2022)
-
A comprehensive prostate biopsy standardization system according to quantitative multiparametric MRI and PSA value: P.R.O.S.T score
World Journal of Urology (2022)
-
Prognostic performance of magnetic resonance imaging-guided biopsy in defining prostate cancer anterior lesions
World Journal of Urology (2021)
-
Necessity of differentiating small (< 10 mm) and large (≥ 10 mm) PI-RADS 4
World Journal of Urology (2020)