Abstract
Background:
Prostate cancer persisting in the primary site after systemic therapy may contribute to emergence of resistance and progression. We previously demonstrated molecular characteristics of lethal cancer in the prostatectomy specimens of patients presenting with lymph node metastasis after chemohormonal treatment. Here we report the post-treatment outcomes of these patients and assess whether a link exists between surgery and treatment-free/cancer-free survival.
Methods:
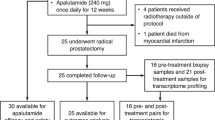
Patients with either clinically detected lymph node metastasis or primaries at high risk for nodal dissemination were treated with androgen ablation and docetaxel. Those responding with PSA concentration <1 ng ml−1 were recommended surgery 1 year from enrollment. ADT was withheld postoperatively. The rate of survival without biochemical progression 1 year after surgery was measured to screen for efficacy.
Results:
Forty patients were enrolled and 39 were evaluable. Three patients (7.7%) declined surgery. Of the remaining 36, 4 patients experienced disease progression during treatment and 4 more did not reach PSA <1. Twenty-six patients (67%) completed surgery, and 13 (33%) were also progression-free 1 year postoperatively (8 with undetectable PSA). With a median follow-up of 61 months, time to treatment failure was 27 months in the patients undergoing surgery. The most frequent patterns of first disease recurrence were biochemical (10 patients) and systemic (5).
Conclusions:
Half of the patients undergoing surgery were off treatment and progression-free 1 year following completion of all therapy. These results suggest that integration of surgery is feasible and may be superior to systemic therapy alone for selected prostate cancer patients presenting with nodal metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Swanson GP, Thompson IM, Basler J . Current status of lymph node-positive prostate cancer: incidence and predictors of outcome. Cancer 2006; 107: 439–450.
Cheng L, Zincke H, Blute ML, Bergstralh EJ, Scherer B, Bostwick DG . Risk of prostate carcinoma death in patients with lymph node metastasis. Cancer 2001; 91: 66–73.
von Bodman C, Godoy G, Chade DC, Cronin A, Tafe LJ, Fine SW et al. Predicting biochemical recurrence-free survival for patients with positive pelvic lymph nodes at radical prostatectomy. J Urol 2010; 184: 143–148.
Hofer MD, Kuefer R, Huang W, Li H, Bismar TA, Perner S et al. Prognostic factors in lymph node-positive prostate cancer. Urology 2006; 67: 1016–1021.
Cheng L, Bergstralh EJ, Cheville JC, Slezak J, Corica FA, Zincke H et al. Cancer volume of lymph node metastasis predicts progression in prostate cancer. Am J Surg Pathol 1998; 22: 1491–1500.
Daneshmand S, Quek ML, Stein JP, Lieskovsky G, Cai J, Pinski J et al. Prognosis of patients with lymph node positive prostate cancer following radical prostatectomy: long-term results. J Urol 2004; 172: 2252–2255.
Briganti A, Karnes JR, Da Pozzo LF, Cozzarini C, Gallina A, Suardi N et al. Two positive nodes represent a significant cut-off value for cancer specific survival in patients with node positive prostate cancer. A new proposal based on a two-institution experience on 703 consecutive N+ patients treated with radical prostatectomy, extended pelvic lymph node dissection and adjuvant therapy. Eur Urol 2009; 55: 261–270.
Wiegand LR, Hernandez M, Pisters LL, Spiess PE . Surgical management of lymph-node-positive prostate cancer: improves symptomatic control. BJU Int 2011; 107: 1238–1242.
Verhagen PC, Schroder FH, Collette L, Bangma CH . Does local treatment of the prostate in advanced and/or lymph node metastatic disease improve efficacy of androgen-deprivation therapy? A systematic review. Eur Urol 2010; 58: 261–269.
Grimm MO, Kamphausen S, Hugenschmidt H, Stephan-Odenthal M, Ackermann R, Vogeli TA . Clinical outcome of patients with lymph node positive prostate cancer after radical prostatectomy versus androgen deprivation. Eur Urol 2002; 41: 628–634.
Boorjian SA, Thompson RH, Siddiqui S, Bagniewski S, Bergstralh EJ, Karnes RJ et al. Long-term outcome after radical prostatectomy for patients with lymph node positive prostate cancer in the prostate specific antigen era. J Urol 2007; 178: 864–870.
Engel J, Bastian PJ, Baur H, Beer V, Chaussy C, Gschwend JE et al. Survival benefit of radical prostatectomy in lymph node-positive patients with prostate cancer. Eur Urol 2010; 57: 754–761.
Steuber T, Budaus L, Walz J, Zorn KC, Schlomm T, Chun F et al. Radical prostatectomy improves progression-free and cancer-specific survival in men with lymph node positive prostate cancer in the prostate-specific antigen era: a confirmatory study. BJU Int 2011; 107: 1755–1761.
Dorin RP, Lieskovsky G, Fairey AS, Cai J, Daneshmand S . Outcomes after radical prostatectomy for patients with clinical stages T1-T2 prostate cancer with pathologically positive lymph nodes in the prostate-specific antigen era. Urol Oncol 2012; 31: 1441–1447.
Schroder FH, Kurth KH, Fossa SD, Hoekstra W, Karthaus PP, De Prijck L et al. Early versus delayed endocrine treatment of T2-T3 pN1-3 M0 prostate cancer without local treatment of the primary tumour: final results of European Organisation for the Research and Treatment of Cancer protocol 30846 after 13 years of follow-up (a randomised controlled trial). Eur Urol 2009; 55: 14–22.
Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol 2006; 7: 472–479.
Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 2009; 373: 301–308.
Mottet N, Peneau M, Mazeron JJ, Molinie V, Richaud P . Addition of radiotherapy to long-term androgen deprivation in locally advanced prostate cancer: an open randomised phase 3 trial. Eur Urol 2012; 62: 213–219.
Tzelepi V, Efstathiou E, Wen S, Troncoso P, Karlou M, Pettaway CA et al. Persistent, biologically meaningful prostate cancer after 1 year of androgen ablation and docetaxel treatment. J Clin Oncol 2011; 29: 2574–2581.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009; 250: 187–196.
Bolla M, van Poppel H, Collette L, van Cangh P, Vekemans K, Da Pozzo L et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911). Lancet 2005; 366: 572–578.
Konety BR, Eastham JA, Reuter VE, Scardino PT, Donat SM, Dalbagni G et al. Feasibility of radical prostatectomy after neoadjuvant chemohormonal therapy for patients with high risk or locally advanced prostate cancer: results of a phase I/II study. J Urol 2004; 171: 709–713.
Febbo PG, Richie JP, George DJ, Loda M, Manola J, Shankar S et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res 2005; 11: 5233–5240.
Dreicer R, Magi-Galluzzi C, Zhou M, Rothaermel J, Reuther A, Ulchaker J et al. Phase II trial of neoadjuvant docetaxel before radical prostatectomy for locally advanced prostate cancer. Urology 2004; 63: 1138–1142.
Chi KN, Chin JL, Winquist E, Klotz L, Saad F, Gleave ME . Multicenter phase II study of combined neoadjuvant docetaxel and hormone therapy before radical prostatectomy for patients with high risk localized prostate cancer. J Urol 2008; 180: 565–570.
Mellado B, Font A, Alcaraz A, Aparicio LA, Veiga FJ, Areal J et al. Phase II trial of short-term neoadjuvant docetaxel and complete androgen blockade in high-risk prostate cancer. Br J Cancer 2009; 101: 1248–1252.
Sweeney C, Chen Y-H, Carducci MA, Liu G, Jarrard DF, Eisenberger MA et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): an ECOG-led phase III randomized trial. J Clin Oncol 2014; 32: 5s.
Aparicio AM, Harzstark AL, Corn PG, Wen S, Araujo JC, Tu SM et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res 2013; 19: 3621–3630.
Acknowledgements
This work was supported in part by the National Cancer Institute at the National Institutes of Health through MD Anderson’s Support Grant P30-CA016672.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Prostate Cancer and Prostatic Diseases website
Supplementary information
Rights and permissions
About this article
Cite this article
Zurita, A., Pisters, L., Wang, X. et al. Integrating chemohormonal therapy and surgery in known or suspected lymph node metastatic prostate cancer. Prostate Cancer Prostatic Dis 18, 276–280 (2015). https://doi.org/10.1038/pcan.2015.23
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2015.23
This article is cited by
-
Neoadjuvant hormonal therapy before radical prostatectomy in high-risk prostate cancer
Nature Reviews Urology (2021)
-
Neoadjuvant Treatment of High-Risk, Clinically Localized Prostate Cancer Prior to Radical Prostatectomy
Current Urology Reports (2016)