Abstract
Background:
Enzalutamide and abiraterone are new androgen-axis disrupting treatments for metastatic castration-resistant prostate cancer (mCRPC). We examined the response and outcomes of enzalutamide-treated mCRPC patients in the real-world context of prior treatments of abiraterone and/or docetaxel.
Methods:
We conducted a seven-institution retrospective study of mCRPC patients treated with enzalutamide between January 2009 and February 2014. We compared the baseline characteristics, PSA declines, PSA progression-free survival (PSA-PFS), duration on enzalutamide and overall survival (OS) across subgroups defined by prior abiraterone and/or docetaxel.
Results:
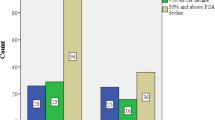
Of 310 patients who received enzalutamide, 36 (12%) received neither prior abiraterone nor prior docetaxel, 79 (25%) received prior abiraterone, 30 (10%) received prior docetaxel and 165 (53%) received both prior abiraterone and prior docetaxel. Within these groups, respectively, ⩾30% PSA decline was achieved among 67, 28, 43 and 24% of patients; PSA-PFS was 5.5 (95% CI 4.2–9.1), 4.0 (3.2–4.8), 4.1 (2.9–5.4) and 2.8 (2.5–3.2) months; median duration of enzalutamide was 9.1 (7.3–not reached), 4.7 (3.7–7.7), 5.4 (3.8–8.4) and 3.9 (3.0–4.6) months. Median OS was reached only for the patients who received both prior abiraterone and docetaxel and was 12.2 months (95% CI 10.7–16.5). 12-month OS was 78% (59–100%), 64% (45–90%), 77% (61–97%) and 51% (41–62%). Of 70 patients who failed to achieve any PSA decline on prior abiraterone, 19 (27%) achieved ⩾30% PSA decline with subsequent enzalutamide.
Conclusions:
The activity of enzalutamide is blunted after abiraterone, after docetaxel, and still more after both, suggesting subsets of overlapping and distinct mechanisms of resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L et al. Abiraterone and increased survival in metastatic prostate cancer. New Engl J Med 2011; 364: 1995–2005.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. New Engl J Med 2012; 367: 1187–1197.
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. New Engl J Med 2013; 368: 138–148.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS et al. Enzalutamide in metastatic prostate cancer before chemotherapy. New Engl J Med 2014; 371: 424–433.
Bianchini D, Lorente D, Rodriguez-Vida A, Omlin A, Pezaro C, Ferraldeschi R et al. Antitumour activity of enzalutamide (MDV3100) in patients with metastatic castration-resistant prostate cancer (CRPC) pre-treated with docetaxel and abiraterone. Eur J Cancer 2014; 50: 78–84.
Badrising S, van der Noort V, van Oort IM, van den Berg HP, Los M, Hamberg P et al. Clinical activity and tolerability of enzalutamide (MDV3100) in patients with metastatic, castration-resistant prostate cancer who progress after docetaxel and abiraterone treatment. Cancer 2013; 120: 968–975.
Thomson D, Charnley N, Parikh O . Enzalutamide after failure of docetaxel and abiraterone in metastatic castrate-resistant prostate cancer. Eur J Cancer 2014; 50: 1040–1041.
Schmid SC, Geith A, Boker A, Tauber R, Seitz AK, Kuczyk M et al. Enzalutamide after docetaxel and abiraterone therapy in metastatic castration-resistant prostate cancer. Adv Ther 2014; 31: 234–241.
Azad AA, Eigl BJ, Murray RN, Kollmannsberger C, Chi KN . Efficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patients. Eur Urol 2014; 67: 23–29.
Ileana E, Loriot Y, Albiges L, Massard C, Blesius A, Di Palma M et al. Abiraterone in patients with metastatic castration-resistant prostate cancer progressing after docetaxel and MDV3100. J Clin Oncology, (Meeting Abstracts) 2012 vol 30 no.15_suppl 4554.
Loriot Y, Bianchini D, Ileana E, Sandhu S, Patrikidou A, Pezaro C et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann Oncol 2013; 24: 1807–1812.
Noonan KL, North S, Bitting RL, Armstrong AJ, Ellard SL, Chi KN . Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol 2013; 24: 1802–1807.
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008; 26: 1148–1159.
Chi KN, San Kheoh T, Ryan CJ, Molina A, Bellmunt J, Vogelzang NJ et al. A prognostic model for predicting overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate after docetaxel. J Clin Oncol; (Meeting Abstracts) 2013 vol 31 no 15_suppl 5013.
Halabi S, Lin CY, Kelly WK, Fizazi KS, Moul JW, Kaplan EB et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol 2014; 32: 671–677.
Armstrong AJ, Tannock IF, de Wit R, George DJ, Eisenberger M, Halabi S . The development of risk groups in men with metastatic castration-resistant prostate cancer based on risk factors for PSA decline and survival. Eur J Cancer 2010; 46: 517–525.
Schrader AJ, Boegemann M, Ohlmann CH, Schnoeller TJ, Krabbe LM, Hajili T et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol 2014; 65: 30–36.
Armstrong AJ, Garrett-Mayer ES, Yang YC, de Wit R, Tannock IF, Eisenberger M . A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res 2007; 13: 6396–6403.
Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 2008; 68: 4447–4454.
Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 2013; 155: 1309–1322.
Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res 2011; 17: 5913–5925.
Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM . Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res 2013; 73: 483–489.
Hu R, Lu C, Mostaghel EA, Yegnasubramanian S, Gurel M, Tannahill C et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res 2012; 72: 3457–3462.
Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide). Cancer Discovery 2013; 3: 1030–1043.
Schweizer MT, Zhou XC, Wang H, Bassi S, Carducci MA, Eisenberger MA et al. The influence of prior abiraterone treatment on the clinical activity of docetaxel in men with metastatic castration-resistant prostate cancer. Eur Urol 2014; 66: 646–652.
van Soest RJ, van Royen ME, de Morree ES, Moll JM, Teubel W, Wiemer EA et al. Cross-resistance between taxanes and new hormonal agents abiraterone and enzalutamide may affect drug sequence choices in metastatic castration-resistant prostate cancer. Eur J Cancer 2013; 49: 3821–3830.
Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 2011; 19: 575–586.
Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. New Engl J Med 2014; 371: 1028–1038.
Azad A, Volik S, Wyatt A, Haegert A, Collins C, Chi KN . Genomic analysis of circulating tumor DNA in plasma of metastatic castration-resistant prostate cancer patients treated with abiraterone acetate and enzalutamide. J Clin Oncol 2014; 32: 5s.
Acknowledgements
This work was supported by the National Cancer Institute at the National Institutes of Health [P50 CA097186 (HHC, RG, EYY), P30 CA006973 (ESA and RN) and T32 CA009515 (HHC)]. We gratefully acknowledge: Maggie So and Kelly Sales for assistance with IRB approvals; Anne Reese, Myan Nguyen, and our clinical colleagues for assistance in identifying patients for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The coauthors declare the following conflicts of interest: AA declares research support from Astellas (Australia) and an honorarium from Janssen (Canada). RBM declares research support from Medivation/Astellas. UNV declares research support and honoraria from Medivation/Astellas. SKP declares honoraria from Medivation/Astellas and is a consultant/advisor for Dendreon. MDG declares research support from Janssen, Dendreon, BioMotiv, is a consultant/advisor to Astellas, Dendreon, BioMotiv and has equity in Dual Therapeutics. ESA is a consultant/advisor to Medivation/Astellas, Janssen Biotech and Sanofi US. KNC is a consultant/advisor to Medivation/Astellas and Janssen Biotech. EYY declares research funding from Bristol-Myers Squibb, Dendreon, GTx, Imclone/Lilly, Janssen and OncoGeneX and honoraria from Bayer, Dendreon, Janssen Biotech, Medivation/Astellas and Sanofi US. All remaining authors have declared no conflicts of interest.
Additional information
Supplementary Information accompanies the paper on the Prostate Cancer and Prostatic Diseases website
Supplementary information
Rights and permissions
About this article
Cite this article
Cheng, H., Gulati, R., Azad, A. et al. Activity of enzalutamide in men with metastatic castration-resistant prostate cancer is affected by prior treatment with abiraterone and/or docetaxel. Prostate Cancer Prostatic Dis 18, 122–127 (2015). https://doi.org/10.1038/pcan.2014.53
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2014.53
This article is cited by
-
Predictive significance of inflammatory markers and mGPS in metastatic castration-resistant prostate cancer treated with abiraterone or enzalutamide
Cancer Chemotherapy and Pharmacology (2024)
-
Editor’ summary: A paradigm shift in castration-resistant prostate cancer management
Prostate Cancer and Prostatic Diseases (2022)
-
Longitudinal model–based meta-analysis for survival probabilities in patients with castration-resistant prostate cancer
European Journal of Clinical Pharmacology (2020)
-
20 years—A retrospective of prostate cancer and prostatic diseases
Prostate Cancer and Prostatic Diseases (2018)
-
Third-line treatment and 177Lu-PSMA radioligand therapy of metastatic castration-resistant prostate cancer: a systematic review
European Journal of Nuclear Medicine and Molecular Imaging (2018)