Abstract
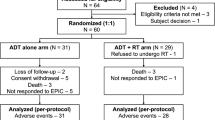
Therapeutic strategy remains unclear with no clear consensus for men with high-risk prostate cancer (PCa) after radical prostatectomy. We aimed to evaluate into a prospective randomized trial the effectiveness and feasibility of adjuvant weekly paclitaxel combined with androgen deprivation therapy (ADT) in these patients. A total of 47 patients with high-risk PCa were randomized 6 weeks after radical prostatectomy: ADT alone versus combination of ADT and weekly paclitaxel. Toxicity, quality-of-life and functional results were compared between the two arms. All 23 patients completed eight cycles of paclitaxel. Toxicity was predominantly of grade 1–2 severity. There were no differences in EORTC QLQ-C30 scores between the two groups and between baseline and last assessment at 24 months after surgery. Urinary continence was complete at 1 year after surgery for all patients and no significant differences were noted at each assessment between the two groups. The interim analysis of this trial confirms the feasibility of weekly paclitaxel in combination with ADT in men at high-risk PCa with curative intent. This adjuvant combined therapy does not alter quality-of-life and continence recovery after surgery plus ADT. A larger cohort is awaited to determine the oncological outcomes of this strategy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ . Cancer statistics. CA Cancer J Clin 2009; 59: 225–249.
Kattan MW, Wheeler TM, Scardino PT . Postoperative nomogram for disease recurrence after prostatectomy for prostate cancer. J Clin Oncol 199; 17: 1499–1507.
Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ . Cancer progression and survival rates following radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol 2004; 172: 910–914.
D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998; 280: 969–974.
Messing E M, Manola J, Sarosdy M, Wilding G, Crawford E D, Trump D . Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med 1999; 341: 1781–1788.
Messing E M, Manola J, Yao J, Kiernan M, Crawford D, Wilding G et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol 2006; 7: 472–479.
McLeod D G, Iversen P, See W A, Morris T, Armstrong J, Wirth M P . Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU Int 2006; 97: 247–254.
Klotz LH, Goldenberg SL, Jewett MA, Fradet Y, Nam R, Barkin J et al. Long-term followup of a randomized trail of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J Urol 2003; 170: 791–794.
Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term follow up of a randomized clinical trial. J Urol 2009; 181: 956–962.
Petrylak DP, Tangen CM, Hussain MH, Lara Jr PN, Jones JA, Taplin ME et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351: 1513–1520.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351 (15): 1502–1512.
Kibel A S, Rosenbaum E, Kattan MW, Picus J, Dreicer R, Klein EA et al. Adjuvant weekly docetaxel for patients with high risk prostate cancer after radical prostatectomy: a multi-institutional pilot study. J Urol 2007; 177: 1777–1781.
Cetnar JP, Malkowicz SB, Palmer SC, Wein AJ, Vaughn DJ . Pilot trial of adjuvant paclitaxel plus estramustine in resected high-risk prostate cancer. Urology 2008; 71: 942–946.
Montgomery B, Lavori P, Garzotto M, Lee K, Brophy M, Thaneemit-Chen S et al. Veterans Affairs Cooperative Studies Program Study 553: Chemotherapy After Prostatectomy, a phase III randomized study of prostatectomy versus prostatectomy with adjuvant docetaxel for patients with high-risk, localized prostate cancer. Urology 2008; 72: 474–480.
Sonpavde G, Chi KN, Powles T, Sweeney CJ, Hahn N, Hutson TE et al. Neoadjuvant therapy followed by prostatectomy for clinically localized prostate cancer. Cancer 2007; 110: 2628–2639.
Dreicer R, Magi-Galluzzi C, Zhou M, Rothaermel J, Reuther A, Ulchaker J et al. Phase II trial of neoadjuvant docetaxel before radical prostatectomy for locally advanced prostate cancer. Urology 2004; 63: 1138–1142.
Febbo PG, Richie JP, George DJ, Loda M, Manola J, Shankar S et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res 2004; 11: 5233–5240.
Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA 2006; 296: 2329–2335.
Sokoloff MH, Rinker-Schaeffer CW, Chung LWK, Brendler CB . Adjunctive therapy for men with high risk localized and locally advanced prostate cancer: targeting disseminated tumor cells. J Urol 2004; 172: 2539–2544.
Roth B J, Yeap B Y, Wilding G, Kasimis B, McLeod D, Loehrer PJ . Taxol in advanced, hormone-refractory carcinoma of the prostate. A phase II trial of the Eastern Cooperative Oncology Group. Cancer 1993; 72: 2457–2460.
Trivedi C, Redman B, Flaherty LE, Kucuk O, Du W, Heilbrun LK et al. Weekly 1-hour infusion of paclitaxel. Clinical feasibility and efficacy in patients with hormone-refractory prostate carcinoma. Cancer 2000; 89: 431–436.
Salomon L, Anastasiadis AG, Katz R, De La Taille A, Saint F, Vordos D et al. Urinary continence and erectile function: a prospective evaluation of functional results after laparoscopic prostatectomy. Eur Urol 2002; 42: 338–343.
Salomon L, Saint F, Anastasiadis AG, Sèbe P, Chopin D, Abbou CC . Combined reporting of cancer control and functional results of radical prostatectomy. Eur Urol 2003; 44: 656–660.
Acknowledgements
With the support of Takeda, Ipsen Biotech and Bristol-Myers-Squibb.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ploussard, G., Paule, B., Salomon, L. et al. Pilot trial of adjuvant paclitaxel plus androgen deprivation for patients with high-risk prostate cancer after radical prostatectomy: results on toxicity, side effects and quality-of-life. Prostate Cancer Prostatic Dis 13, 97–101 (2010). https://doi.org/10.1038/pcan.2009.51
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2009.51
Keywords
This article is cited by
-
Radical Prostatectomy as Primary Treatment of High-risk Prostate Cancer
Current Urology Reports (2012)