Abstract
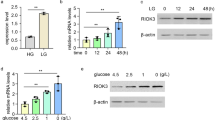
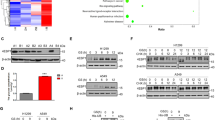
Metabolic stress is a common phenomenon in solid tumors. Compensatory mechanisms to overcome metabolic stress (glucose deprivation) are vital to tumor cell survival. The histone demethylase Jumonji domain-containing protein 2B (JMJD2B) is vital for the growth and progression of various cancers. However, the role of JMJD2B during glucose deprivation remains unclear. Our aim was to examine the function of JMJD2B in glucose-deprived colon cancer cells and the involvement of extracellular signal-regulated kinase (ERK) and glucose transporter 1 (GLUT1). Our study demonstrated that JMJD2B expression was upregulated via ERK phosphorylation during glucose deprivation. Further, the cell viability assay showed that the effect of p-ERK on the viability of colon cancer cells was at least partly dependent on JMJD2B expression. Glucose deprivation increased interaction between JMJD2B and p-ERK, as demonstrated by the co-immunoprecipitation and fluorescence assays. Glucose deprivation also resulted in phosphorylation of JMJD2B by p-ERK, as shown by the immunoprecipitation assay and western blotting analysis. In addition, the phosphorylation of JMJD2B via p-ERK at Thr305, Ser352, Ser566 and Thr1065 contribute to JMJD2B stability. p-ERK stabilizes the JMJD2B protein level by protecting JMJD2B from ubiquitination and proteasome degradation. We found that knockdown of JMJD2B significantly impaired colon cancer cell viability, which is accompanied by a significant reduction in glucose uptake and lactate production. Furthermore, knockdown of JMJD2B after glucose deprivation caused decreased level of GLUT1 through increasing H3K9 tri-methylation levels on its promoter, demonstrated by chromatin immunoprecipitation assay. Moreover, targeting JMJD2B in xenograft tumors also decreased the GLUT1 level. Finally, a positive correlation was observed between p-ERK, JMJD2B and GLUT1 expression in 85 human colon cancer tissue specimens. These results indicated that p-ERK-mediated phosphorylation and stabilization of JMJD2B during glucose deprivation contributes to its role in glucose uptake and cell viability, which may be modulated through epigenetically upregulation of GLUT1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Musselman CA, Lalonde ME, Côté J, Kutateladz TG . Perceiving the epigenetic landscape through histone readers. Nat Struct Mol Biol 2012; 19: 1218–1227.
Bannister AJ, Kouzarides T . Regulation of chromatin by histone modifications. Cell Res 2011; 21: 381–395.
Greer EL, Shi Y . Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet 2012; 13: 343–357.
Martin C, Zhang Y . The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol 2005; 6: 838–849.
Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 2004; 119: 941–953.
Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature 2006; 439: 811–816.
Katoh M, Katoh M . Identification and characterization of JMJD2 family genes in silico. Int J Oncol 2004; 24: 1623–1628.
Shi L, Sun L, Li Q, Liang J, Yu W, Yi X et al. Histone demethylase JMJD2B coordinates H3K4/H3K9 methylation and promotes hormonally responsive breast carcinogenesis. Proc Natl Acad Sci USA 2011; 108: 7541–7546.
Kawazu M, Saso K, Tong KI, McQuire T, Goto K, Son DO et al. Histone demethylase JMJD2B functions as a co-factor of estrogen receptor in breast cancer proliferation and mammary gland development. PLoS ONE 2011; 6: e17830.
Toyokawa G, Cho HS, Iwai Y, Yoshimatsu M, Takawa M, Hayami S et al. The histone demethylase JMJD2B plays an essential role in human carcinogenesis through positive regulation of cyclin-dependent kinase 6. Cancer Prev Res (Phila) 2011; 4: 2051–2061.
Zhao L, Li W, Zang W, Liu Z, Xu X, Yu H et al. JMJD2B promotes epithelial-mesenchymal transition by cooperating with β-catenin and enhances gastric cancer metastasis. Clin Cancer Res 2013; 19: 6419–6429.
Beyer S, Kristensen MM, Jensen KS, Johansen JV, Staller P . The histone demethylases JMJD1A and JMJD2B are transcriptional targets of hypoxia-inducible factor HIF. J Biol Chem 2008; 283: 36542–36552.
Fu L, Chen L, Yang J, Ye T, Chen Y, Fang J . HIF-1α-induced histone demethylase JMJD2B contributes to the malignant phenotype of colorectal cancer cells via an epigenetic mechanism. Carcinogenesis 2012; 33: 1664–1673.
Chen L, Fu L, Kong X, Xu J, Wang Z, Ma X et al. Jumonji domain-containing protein 2B silencing induces DNA damage response via STAT3 pathway in colorectal cancer. Br J Cancer 2014; 110: 1014–1026.
Cairns RA, Harris IS, Mak TW . Regulation of cancer cell metabolism. Nat Rev Cancer 2011; 11: 85–95.
Folkman J . Angiogenesis and apoptosis. Semin Cancer Biol 2003; 13: 159–167.
Graham NA, Tahmasian M, Kohli B, Komisopoulou E, Zhu M, Vivanco I et al. Glucose deprivation activates a metabolic and signaling amplification loop leading to cell death. Mol Syst Biol 2012; 8: 589.
Chen XQ, Lau LT, Fung YW, Yu AC . Inactivation of bad by site-specific phosphorylation: the checkpoint for ischemic astrocytes to initiate or resist apoptosis. J Neurosci Res 2005; 79: 798–808.
Ciccarelli R, D'Alimonte I, Ballerini P, D'Auro M, Nargi E, Buccella S et al. Molecular signalling mediating the protective effect of A1 adenosine and mGlu3 metabotropic glutamate receptor activation against apoptosis by oxygen/glucose deprivation in cultured astrocytes. Mol Pharmacol 2007; 71: 1369–1380.
Eckert A, Böck BC, Tagscherer KE, Haas TL, Grund K, Sykora J et al. The PEA-15/PED protein protects glioblastoma cells from glucose deprivation-induced apoptosis via the ERK/MAP kinase pathway. Oncogene 2008; 27: 1155–1166.
Flier JS, Mueckler MM, Usher P, Lodish HF . Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science 1987; 235: 1492–1495.
Malumbres M, Barbacid M . RAS oncogenes: the first 30 years. Nat Rev Cancer 2003; 3: 459–465.
Tateishi K, Okada Y, Kallin EM, Zhang Y . Role of Jhdm2a in regulating metabolic gene expression and obesity resistance. Nature 2009; 458: 757–761.
Zhao E, Ding J, Xia Y, Liu M, Ye B, Choi JH et al. KDM4C and ATF4 cooperate in transcriptional control of amino acid metabolism. Cell Rep 2016; 14: 506–519.
Roskoski R Jr . ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res 2012; 66: 105–143.
Kim MO, Kim SH, Cho YY, Nadas J, Jeong CH, Yao K et al. ERK1 and ERK2 regulate embryonic stem cell self-renewal through phosphorylation of Klf4. Nat Struct Mol Biol 2012; 19: 283–290.
Kim SH, Kim MO, Cho YY, Yao K, Kim DJ, Jeong CH et al. ERK1 phosphorylates Nanog to regulate protein stability and stem cell self-renewal. Stem Cell Res 2014; 13: 1–11.
Wu N, Zheng B, Shaywitz A, Dagon Y, Tower C, Bellinger G et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol Cell 2013; 49: 1167–1175.
Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H et al. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res 2009; 69: 4918–4925.
Ma L, Tao Y, Duran A, Llado V, Galvez A, Barger JF, Castilla EA et al. Control of nutrient stress-induced metabolic reprogramming by PKCζ in tumorigenesis. Cell 2013; 152: 599.
Lee YJ, Galoforo SS, Berns CM, Chen JC, Davis BH, Sim JE et al. Glucose deprivation-induced cytotoxicity and alterations in mitogen-activated protein kinase activation are mediated by oxidative stress in multidrug-resistant human breast carcinoma cells. J Biol Chem 1998; 273: 5294–5299.
Mendoza MC, Er EE, Blenis J . The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 2011; 36: 320–328.
Dhillon AS, Hagan S, Rath O, Kolch W . MAP kinase signalling pathways in cancer. Oncogene 2007; 26: 3279–3290.
Través PG, de Atauri P, Marín S, Pimentel-Santillana M, Rodríguez-Prados JC, Marín de Mas I et al. Relevance of the MEK/ERK signaling pathway in the metabolism of activated macrophages: a metabolomic approach. J Immunol 2012; 188: 1402–1410.
Bramanti V, Grasso S, Tibullo D, Giallongo C, Pappa R, Brundo MV et al. Neuroactive molecules and growth factors modulate cytoskeletal protein expression during astroglial cell proliferation and differentiation in culture. J Neurosci Res 2016; 94: 90–98.
Yang SH, Sharrocks AD, Whitmarsh AJ . Transcriptional regulation by the MAP kinase signaling cascades. Gene 2003; 320: 3–21.
Whitmarsh AJ . Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim Biophys Acta 2007; 1773: 1285–1298.
Baek SH . When signaling kinases meet histones and histone modifiers in the nucleus. Mol Cell 2011; 42: 274–284.
Madak-Erdogan Z, Lupien M, Stossi F, Brown M, Katzenellenbogen BS . Genomic collaboration of estrogen receptor alpha and extracellular signal-regulated kinase 2 in regulating gene and proliferation programs. Mol Cell Biol 2011; 31: 226–236.
Cheng MB, Zhang Y, Cao CY, Zhang WL, Zhang Y, Shen YF . Specific phosphorylation of histone demethylase KDM3A determines target gene expression in response to heat shock. PLoS Biol 2014; 12: e1002026.
Liang CY, Hsu PH, Chou DF, Pan CY, Wang LC, Huang WC et al. The histone H3K36 demethylase Rph1/KDM4 regulates the expression of the photoreactivation gene PHR1. Nucleic Acids Res 2011; 39: 4151–4165.
Badeaux AI, Shi Y . Emerging roles for chromatin as a signal integration and storage platform. Nat Rev Mol Cell Biol 2013; 14: 211–224.
Mayya V, Lundgren DH, Hwang SI, Rezaul K, Wu L, Eng JK et al. Quantitative phosphoproteomic analysis of T cell receptor signaling reveals system-wide modulation of protein-protein interactions. Sci Signal 2009; 2: ra46.
Hong J, Zhou J, Fu J, He T, Qin J, Wang L et al. Phosphorylation of serine 68 of Twist1 by MAPKs stabilizes Twist1 protein and promotes breast cancer cell invasiveness. Cancer Res 2011; 71: 3980–3990.
Yamashita M, Shinnakasu R, Asou H, Kimura M, Hasegawa A, Hashimoto K et al. Ras-ERK MAPK cascade regulates GATA3 stability and Th2 differentiation through ubiquitin-proteasome pathway. J Biol Chem 2005; 280: 29409–29419.
Ipenberg I, Guttmann-Raviv N, Khoury HP, Kupershmit I, Ayoub N . Heat shock protein 90 (Hsp90) selectively regulates the stability of KDM4B/JMJD2B histone demethylase. J Biol Chem 2013; 288: 14681–14687.
Ganapathy-Kanniappan S, Geschwind JF . Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer 2013; 12: 152.
Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science 2009; 325: 1555–1559.
Huang C, Sheng S, Li R, Sun X, Liu J, Huang G . Lactate promotes resistance to glucose starvation via upregulation of Bcl-2 mediated by mTOR activation. Oncol Rep 2015; 33: 875–884.
Macheda ML, Rogers S, Best JD . Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol 2005; 202: 654–662.
Calvo MB, Figueroa A, Pulido EG, Campelo RG, Aparicio LA . Potential role of sugar transporters in cancer and their relationship with anticancer therapy. Int J Endocrinol 2010; 2010: pii: 205357.
Cloos PA, Christensen J, Agger K, Maiolica A, Rappsilber J, Antal T et al. The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature 2006; 442: 307–311.
Acknowledgements
This project was supported by the National Key Technology R&D Program (No. 2014BAI09B05), the National Natural Science Foundation of China (No. 81572303 to CYX, No. 81302085 to GQY and No. 81530072 to FJY), Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (No. 20152210) & Program of Shanghai Academic/Technology Research Leader (No. 15XD1502600).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Fu, LN., Wang, YQ., Tan, J. et al. Role of JMJD2B in colon cancer cell survival under glucose-deprived conditions and the underlying mechanisms. Oncogene 37, 389–402 (2018). https://doi.org/10.1038/onc.2017.345
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.345
This article is cited by
-
Metabolic reprogramming in colorectal cancer: regulatory networks and therapy
Cell & Bioscience (2023)
-
The phosphorylation to acetylation/methylation cascade in transcriptional regulation: how kinases regulate transcriptional activities of DNA/histone-modifying enzymes
Cell & Bioscience (2022)
-
KDM4B facilitates colorectal cancer growth and glucose metabolism by stimulating TRAF6-mediated AKT activation
Journal of Experimental & Clinical Cancer Research (2020)
-
The role of histone methylation in the development of digestive cancers: a potential direction for cancer management
Signal Transduction and Targeted Therapy (2020)
-
Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials
Signal Transduction and Targeted Therapy (2019)