Abstract
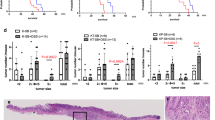
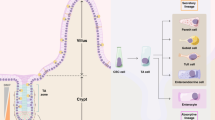
Mutations in mismatch repair (MMR) genes result in microsatellite instability (MSI) and early onset of colorectal cancer. To get mechanistic insights into the time scale, sequence and frequency of intestinal stem cell (ISC) transformation, we quantified MSI and growth characteristics of organoids of Msh2-deficient and control mice from birth until tumor formation and related them to tissue gene expression. Although in Msh2-deficient organoids MSI continuously increased from birth, growth characteristics remained stable at first. Months before tumor onset, normal Msh2-deficient tissue contained tumor precursor cells forming organoids with higher MSI, cystic growth and growth rates resembling temporarily those of tumor organoids. Consistently, Msh2-deficient tissue exhibited a tumor-like gene signature. Normal Msh2-deficient organoids showed increased inheritable transient cyst-like growth, which became independent of R-spondin. ISC transformation proceeded faster in vitro than in vivo independent of the underlying genotype but more under MMR deficiency. Transient cyst-like growth but not MSI was suppressed by aspirin. In summary, as highlighted by organoids, molecular alterations continuously proceeded long before tumor onset in MMR-deficient intestine, thus increasing its susceptibility for ISC transformation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Giardiello FM, Allen JI, Axilbund JE, Boland CR, Burke CA, Burt RW et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-Society Task Force on colorectal cancer. Gastroenterology 2014; 147: 502–526.
Lynch HT, Snyder CL, Shaw TG, Heinen CD, Hitchins MP . Milestones of Lynch syndrome: 1895-2015. Nat Rev Cancer 2015; 15: 181–194.
Bhalla A, Zulfiqar M, Weindel M, Shidham VB . Molecular diagnostics in colorectal carcinoma. Clin Lab Med 2013; 33: 835–859.
Kloor M, Huth C, Voigt AY, Benner A, Schirmacher P, von Knebel DM et al. Prevalence of mismatch repair-deficient crypt foci in Lynch syndrome: a pathological study. Lancet Oncol 2012; 13: 598–606.
Staffa L, Echterdiek F, Nelius N, Benner A, Werft W, Lahrmann B et al. Mismatch repair-deficient crypt foci in Lynch syndrome—molecular alterations and association with clinical parameters. PLoS One 2015; 10: e0121980.
Sato T, Vries RG, Snippert HJ, van de WM, Barker N, Stange DE et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009; 459: 262–265.
Sato T, Clevers H . Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 2013; 340: 1190–1194.
Kucherlapati MH, Lee K, Nguyen AA, Clark AB, Hou H Jr, Rosulek A et al. An Msh2 conditional knockout mouse for studying intestinal cancer and testing anticancer agents. Gastroenterology 2010; 138: 993–1002.
McIlhatton MA, Tyler J, Kerepesi LA, Bocker-Edmonston T, Kucherlapati MH, Edelmann W et al. Aspirin and low-dose nitric oxide-donating aspirin increase life span in a Lynch syndrome mouse model. Cancer Prev Res 2011; 4: 684–693.
Kabbarah O, Mallon MA, Pfeifer JD, Edelmann W, Kucherlapati R, Goodfellow PJ . A panel of repeat markers for detection of microsatellite instability in murine tumors. Mol Carcinog 2003; 38: 155–159.
Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011; 469: 415–418.
Sato T, Stange DE, Ferrante M, Vries RG, van Es JH, van den Brink S et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 2011; 141: 1762–1772.
Germann M, Xu H, Malaterre J, Sampurno S, Huyghe M, Cheasley D et al. Tripartite interactions between Wnt signaling, Notch and Myb for stem/progenitor cell functions during intestinal tumorigenesis. Stem Cell Res 2014; 13: 355–366.
Harris AR, Daeden A, Charras GT . Formation of adherens junctions leads to the emergence of a tissue-level tension in epithelial monolayers. J Cell Sci 2014; 127: 2507–2517.
Finkbeiner SR, Hill DR, Altheim CH, Dedhia PH, Taylor MJ, Tsai YH et al. Transcriptome-wide analysis reveals hallmarks of human intestine development and maturation in vitro and in vivo. Stem Cell Rep 2015. doi:10.1016/j.stemcr.2015.04.010.
Fre S, Pallavi SK, Huyghe M, Lae M, Janssen KP, Robine S et al. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc Natl Acad Sci USA 2009; 106: 6309–6314.
Genander M, Halford MM, Xu NJ, Eriksson M, Yu Z, Qiu Z et al. Dissociation of EphB2 signaling pathways mediating progenitor cell proliferation and tumor suppression. Cell 2009; 139: 679–692.
Rowland BD, Peeper DS . Peeper DS. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer 2006; 6: 11–23.
Martinez P, Siegl-Cachedenier I, Flores JM, Blasco MA . MSH2 deficiency abolishes the anticancer and pro-aging activity of short telomeres. Aging Cell 2009; 8: 2–17.
Munzy DM, Bainbridge MN, Chang K, Dinh HH, Drummond JA, Fowler G et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012; 487: 330–337.
Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015; 21: 1350–1356.
Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M et al. Genetic alterations during colorectal-tumor development. N Engl J Med 1988; 319: 525–532.
Fearon ER, Vogelstein B . A genetic model for colorectal tumorigenesis. Cell 1990; 61: 759–767.
Barker N, Ridgway RA, van Es JH, van de WM, Begthel H, van den BM et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009; 457: 608–611.
Woerner SM, Tosti E, Yuan YP, Kloor M, Bork P, Edelmann W et al. Detection of coding microsatellite frameshift mutations in DNA mismatch repair-deficient mouse intestinal tumors. Mol Carcinog 2015; 54: 1376–1386.
Lu Y, Soong TD, Elemento O . A novel approach for characterizing microsatellite instability in cancer cells. PLoS One 2013; 8: e63056.
Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 2013; 13: 653–658.
Schuijers J, Junker JP, Mokry M, Hatzis P, Koo BK, Sasselli V et al. Ascl2 acts as an R-spondin/Wnt-responsive switch to control stemness in intestinal crypts. Cell Stem Cell 2015; 16: 158–170.
Przybilla J, Rohlf T, Loeffler M, Galle J . Understanding epigenetic changes in aging stem cells—a computational model approach. Aging Cell 2014; 13: 320–328.
Tian H, Biehs B, Chiu C, Siebel CW, Wu Y, Costa M et al. Opposing activities of Notch and Wnt signaling regulate intestinal stem cells and gut homeostasis. Cell Rep 2015; 11: 33–42.
Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van BR, Buijs A et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature 2015; 521: 43–47.
Holmberg J, Genander M, Halford MM, Anneren C, Sondell M, Chumley MJ et al. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell 2006; 125: 1151–1163.
Ghaleb AM, McConnell BB, Kaestner KH, Yang VW . Altered intestinal epithelial homeostasis in mice with intestine-specific deletion of the Kruppel-like factor 4 gene. Dev Biol 2011; 349: 310–320.
Vermeulen L, Snippert HJ . Stem cell dynamics in homeostasis and cancer of the intestine. Nat Rev Cancer 2014; 14: 468–480.
Tao S, Tang D, Morita Y, Sperka T, Omrani O, Lechel A et al. Wnt activity and basal niche position sensitize intestinal stem and progenitor cells to DNA damage. EMBO J 2015; 34: 624–640.
Burn J, Gerdes AM, Macrae F, Mecklin JP, Moeslein G, Olschwang S et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011; 378: 2081–2087.
Kortum B, Campregher C, Lang M, Khare V, Pinter M, Evstatiev R et al. Mesalazine and thymoquinone attenuate intestinal tumour development in Msh2loxP/loxP Villin-Cre mice. Gut 2015; 64: 1905–1912.
Heijmans J, van Lidth de Jeude JF, Koo BK, Rosekrans SL, Wielenga MC, van de Wetering M et al. ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Rep 2013; 3: 1128–1139.
Cromer A, Carles A, Millon R, Ganguli G, Chalmel F, Lemaire F et al. Identification of genes associated with tumorigenesis and metastatic potential of hypopharyngeal cancer by microarray analysis. Oncogene 2004; 23: 2484–2498.
Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M, Clevers H . Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology 2005; 129: 626–638.
Acknowledgements
The study was supported by the BMBF grants HNPCC-Sys (grant number: 031 6065A) and INDRA (grant number: 031A312).
Author contributions
Study concept and design: GA. Acquisition of data: KK1, TK, KR and CK. Analysis and interpretation of data: KR, JP, JG and GA. Technical support: KK2, PB, HLW and ML. Drafting of the manuscript: JG and GA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Keysselt, K., Kreutzmann, T., Rother, K. et al. Different in vivo and in vitro transformation of intestinal stem cells in mismatch repair deficiency. Oncogene 36, 2750–2761 (2017). https://doi.org/10.1038/onc.2016.429
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.429