Abstract
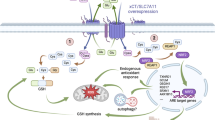
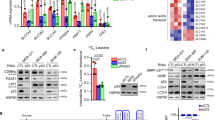
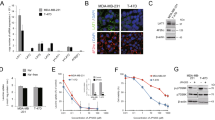
Despite the advances in the diagnosis and treatment of breast cancer, breast cancers still cause significant mortality. For some patients, especially those with triple-negative breast cancer, current treatments continue to be limited and ineffective. Therefore, there remains an unmet need for a novel therapeutic approach. One potential strategy is to target the altered metabolic state that is rewired by oncogenic transformation. Specifically, this rewiring may render certain outside nutrients indispensable. To identify such a nutrient, we performed a nutrigenetic screen by removing individual amino acids to identify possible addictions across a panel of breast cancer cells. This screen revealed that cystine deprivation triggered rapid programmed necrosis, but not apoptosis, in the basal-type breast cancer cells mostly seen in TNBC tumors. In contrast, luminal-type breast cancer cells are cystine-independent and exhibit little death during cystine deprivation. The cystine addiction phenotype is associated with a higher level of cystine-deprivation signatures noted in the basal type breast cancer cells and tumors. We found that the cystine-addicted breast cancer cells and tumors have strong activation of TNFα and MEKK4-p38-Noxa pathways that render them susceptible to cystine deprivation-induced necrosis. Consistent with this model, silencing of TNFα and MEKK4 dramatically reduces cystine-deprived death. In addition, the cystine addiction phenotype can be abrogated in the cystine-addictive cells by miR-200c, which converts the mesenchymal-like cells to adopt epithelial features. Conversely, the introduction of inducers of epithelial-mesenchymal transition (EMT) in cystine-independent breast cancer cells conferred the cystine-addiction phenotype by modulating the signaling components of cystine addiction. Together, our data reveal that cystine-addiction is associated with EMT in breast cancer during tumor progression. These findings provide the genetic and mechanistic basis to explain how cystine deprivation triggers necrosis by activating pre-existing oncogenic pathways in cystine-addicted TNBC with prominent mesenchymal features.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
27 July 2017
This article has been corrected since Advance Online Publication and a corrigendum is also printed in this issue.
References
Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M et alSurveillance Modeling Network C. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005; 353: 1784–1792.
Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature 2012; 490: 61–70.
Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009; 457: 910–914.
Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2010; 465: 966.
Hakimi AA, Reznik E, Lee CH, Creighton CJ, Brannon AR, Luna A et al. An integrated metabolic atlas of clear cell renal cell carcinoma. Cancer Cell 2016; 29: 104–116.
Tang X, Lin CC, Spasojevic I, Iversen E, Chi JT, Marks JR . A joint analysis of metabolomics and genetics of breast cancer. Breast Cancer Res 2014; 16: 415.
Keenan MM, Chi JT . Alternative fuels for cancer cells. Cancer J 2015; 21: 49–55.
Song CW, Clement JJ, Levitt SH . Preferential cytotoxicity of 5-thio-D-glucose against hypoxic tumor cells. J Natl Cancer Inst 1976; 57: 603–605.
Lampidis TJ, Kurtoglu M, Maher JC, Liu H, Krishan A, Sheft V et al. Efficacy of 2-halogen substituted D-glucose analogs in blocking glycolysis and killing 'hypoxic tumor cells'. Cancer Chemother Pharmacol 2006; 58: 725–734.
Gatza ML, Kung HN, Blackwell KL, Dewhirst MW, Marks JR, Chi JT . Analysis of tumor environmental response and oncogenic pathway activation identifies distinct basal and luminal features in HER2-related breast tumor subtypes. Breast Cancer Res 2011; 13: R62.
Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y . Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol 2007; 178: 93–105.
Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA 2008; 105: 18782–18787.
Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 2009; 458: 762–765.
Yang C, Sudderth J, Dang T, Bachoo RM, McDonald JG, DeBerardinis RJ . Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res 2009; 69: 7986–7993.
Seltzer MJ, Bennett BD, Joshi AD, Gao P, Thomas AG, Ferraris DV et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res 2010; 70: 8981–8987.
Kung HN, Marks JR, Chi JT . Glutamine synthetase is a genetic determinant of cell type-specific glutamine independence in breast epithelia. PLoS Genet 2011; 7: e1002229.
Sheen JH, Zoncu R, Kim D, Sabatini DM . Defective regulation of autophagy upon leucine deprivation reveals a targetable liability of human melanoma cells in vitro and in vivo. Cancer Cell 2011; 19: 613–628.
Scott L, Lamb J, Smith S, Wheatley DN . Single amino acid (arginine) deprivation: rapid and selective death of cultured transformed and malignant cells. Br j cancer 2000; 83: 800–810.
Kreis W, Baker A, Ryan V, Bertasso A . Effect of nutritional and enzymatic methionine deprivation upon human normal and malignant cells in tissue culture. Cancer Res 1980; 40: 634–641.
Ohtawa K, Ueno T, Mitsui K, Kodera Y, Hiroto M, Matsushima A et al. Apoptosis of leukemia cells induced by valine-deficient medium. Leukemia 1998; 12: 1651–1652.
Terunuma A, Putluri N, Mishra P, Math, xE A. E, Dorsey TH, Yi M et al. MYC-driven accumulation of 2-hydroxyglutarate is associated with breast cancer prognosis. J Clin Invest 2014; 124: 398–412.
Timmerman LA, Holton T, Yuneva M, Louie RJ, Padro M, Daemen A et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell 2013; 24: 450–465.
Tang X, Keenan MM, Wu J, Lin CA, Dubois L, Thompson JW et al. Comprehensive profiling of amino acid response uncovers unique methionine-deprived response dependent on intact creatine biosynthesis. PLoS genet 2015; 11: e1005158.
Tang X, Wu J, Ding C-K, Lu M, Keenan MM, Lin C-C et al. Cystine deprivation triggers programmed necrosis in VHL-deficient renal cell carcinomas. Cancer Res 2016; 76: 1892–1903.
Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006; 10: 515–527.
Murphy JM, Silke J . Ars Moriendi; the art of dying well—new insights into the molecular pathways of necroptotic cell death. EMBO rep 2014; 15: 155–164.
Gatza ML, Lucas JE, Barry WT, Kim JW, Wang Q, Crawford MD et al. A pathway-based classification of human breast cancer. Proc Natl Acad Sci USA 2010; 107: 6994–6999.
Takekawa M, Posas F, Saito H . A human homolog of the yeast Ssk2/Ssk22 MAP kinase kinase kinases, MTK1, mediates stress-induced activation of the p38 and JNK pathways. EMBO J 1997; 16: 4973–4982.
Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, Takekawa M . Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat Cell Biol 2008; 10: 1324–1332.
Chen JL, Merl D, Peterson CW, Wu J, Liu PY, Yin H et al. Lactic acidosis triggers starvation response with paradoxical induction of TXNIP through MondoA. PLoS genet 2010; 6: e1001093.
Lucas JE, Kung HN, Chi JT . Latent factor analysis to discover pathway-associated putative segmental aneuploidies in human cancers. PLoS Comput Biol 2010; 6: e1000920.
Chin K, DeVries S, Fridlyand J, Spellman PT, Roydasgupta R, Kuo WL et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell 2006; 10: 529–541.
Asiedu MK, Ingle JN, Behrens MD, Radisky DC, Knutson KL . TGFbeta/TNF(alpha)-mediated epithelial-mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res 2011; 71: 4707–4719.
Ho M-Y, Tang S-J, Chuang M-J, Cha T-L, Li J-Y, Sun G-H et al. TNF-α induces epithelial–mesenchymal transition of renal cell carcinoma cells via a GSK3β-dependent mechanism. Mol Cancer Res 2012; 10: 1109–1119.
Harten SK, Shukla D, Barod R, Hergovich A, Balda MS, Matter K et al. Regulation of renal epithelial tight junctions by the von Hippel-Lindau tumor suppressor gene involves occludin and claudin 1 and is independent of E-cadherin. Mol Biol Cell 2009; 20: 1089–1101.
Zhao W, Prijic S, Urban BC, Tisza MJ, Zuo Y, Li L et al. Candidate anti-metastasis drugs suppress the metastatic capacity of breast cancer cells by reducing membrane fluidity. Cancer Res 2016; 76: 2037–2049.
Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL et al. Epithelial-mesenchymal transition induced by TNF-alpha requires NF-kappaB-mediated transcriptional upregulation of Twist1. Cancer Res 2012; 72: 1290–1300.
Zhang X, Zhao X, Shao S, Zuo X, Ning Q, Luo M et al. Notch1 induces epithelial-mesenchymal transition and the cancer stem cell phenotype in breast cancer cells and STAT3 plays a key role. Int J Oncol 2015; 46: 1141–1148.
Kulbe H, Thompson R, Wilson JL, Robinson S, Hagemann T, Fatah R et al. The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res 2007; 67: 585–592.
Wu Y, Zhou BP . TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br j cancer 2010; 102: 639–644.
Paranjape AN, Soundararajan R, Werden SJ, Joseph R, Taube JH, Liu H et al. Inhibition of FOXC2 restores epithelial phenotype and drug sensitivity in prostate cancer cells with stem-cell properties. Oncogene e-pub ahead of print 25 January 2016 doi:10.1038/onc.2015.498).
Yae T, Tsuchihashi K, Ishimoto T, Motohara T, Yoshikawa M, Yoshida GJ et al. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat Commun 2012; 3: 883.
Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387–400.
Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013; 369: 722–731.
Foulkes WD, Smith IE, Reis-Filho JS . Triple-negative breast cancer. N Engl J Med 2010; 363: 1938–1948.
Yoshikawa M, Tsuchihashi K, Ishimoto T, Yae T, Motohara T, Sugihara E et al. xCT inhibition depletes CD44v-expressing tumor cells that are resistant to EGFR-targeted therapy in head and neck squamous cell carcinoma. Cancer Res 2013; 73: 1855–1866.
Godwin AK, Meister A, O'Dwyer PJ, Huang CS, Hamilton TC, Anderson ME . High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci USA 1992; 89: 3070–3074.
Moore T, Le A, Niemi AK, Kwan T, Cusmano-Ozog K, Enns GM et al. A new LC-MS/MS method for the clinical determination of reduced and oxidized glutathione from whole blood. J Chromatogr B Analyt Technol Biomed Life Sci 2013; 929: 51–55.
Keenan MM, Liu B, Tang X, Wu J, Cyr D, Stevens RD et al. ACLY and ACC1 regulate hypoxia-induced apoptosis by modulating ETV4 via alpha-ketoglutarate. PLoS Genet 2015; 11: e1005599.
Acknowledgements
We wish to acknowledge the financial support from the NIH (CA125618 and CA106520 to JTC) and Department of Defense (W81XWH-12-1-0148, W81XWH-14-1-0309, W81XWH-15-1-0486 to JTC). We appreciate the technical assistance of Ivan Spasojevic and the DCI’s Pharmacokinetics and Investigational Chemotherapy share facility. We are also grateful to members of Chi lab for critical discussions and editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Tang, X., Ding, CK., Wu, J. et al. Cystine addiction of triple-negative breast cancer associated with EMT augmented death signaling. Oncogene 36, 4235–4242 (2017). https://doi.org/10.1038/onc.2016.394
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.394
This article is cited by
-
MAP3K4 kinase action and dual role in cancer
Discover Oncology (2024)
-
Oxidative stress regulation and related metabolic pathways in epithelial–mesenchymal transition of breast cancer stem cells
Stem Cell Research & Therapy (2023)
-
Post-translational modifications reshape the antigenic landscape of the MHC I immunopeptidome in tumors
Nature Biotechnology (2023)
-
Development of doxorubicin hydrochloride–loaded whey protein nanoparticles and its surface modification with N-acetyl cysteine for triple-negative breast cancer
Drug Delivery and Translational Research (2022)
-
HDAC6 inhibitors sensitize non-mesenchymal triple-negative breast cancer cells to cysteine deprivation
Scientific Reports (2021)