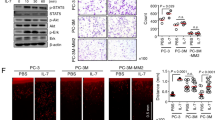
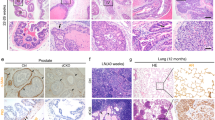
Abstract
Chronic inflammation has been associated with a variety of human cancers including prostate cancer. Interleukin-17 (IL-17) is a critical pro-inflammatory cytokine, which has been demonstrated to promote development of prostate cancer, colon cancer, skin cancer, breast cancer, lung cancer and pancreas cancer. IL-17 promotes prostate adenocarcinoma with a concurrent increase of matrix metalloproteinase 7 (MMP7) expression in mouse prostate. Whether MMP7 mediates IL-17’s action and the underlying mechanisms remain unknown. We generated Mmp7 and Pten double knockout (KO) (Mmp7−/−) mouse model and demonstrated that MMP7 promotes prostate adenocarcinoma through induction of epithelial-to-mesenchymal transition (EMT) in Pten-null mice. MMP7 disrupted E-cadherin/β-catenin complex to upregulate EMT transcription factors in mouse prostate tumors. IL-17 receptor C and Pten double KO mice recapitulated the weak EMT characteristics observed in Mmp7−/− mice. IL-17 induced MMP7 and EMT in human prostate cancer LNCaP, C4-2B and PC-3 cell lines, while small interfering RNA knockdown of MMP7 inhibited IL-17-induced EMT. Compound III, a selective MMP7 inhibitor, decreased development of invasive prostate cancer in Pten single KO mice. In human normal prostates and prostate tumors, IL-17 mRNA levels were positively correlated with MMP7 mRNA levels. These findings demonstrate that MMP7 mediates IL-17’s function in promoting prostate carcinogenesis through induction of EMT, indicating IL-17–MMP7–EMT axis as a potential target for developing new strategies in the prevention and treatment of prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Coussens LM, Werb Z . Inflammation and cancer. Nature 2002; 420: 860–867.
Nickel JC, Downey J, Young I, Boag S . Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int 1999; 84: 976–981.
Gerstenbluth RE, Seftel AD, MacLennan GT, Rao RN, Corty EW, Ferguson K et al. Distribution of chronic prostatitis in radical prostatectomy specimens with up-regulation of bcl-2 in areas of inflammation. J Urol 2002; 167: 2267–2270.
Schatteman PH, Hoekx L, Wyndaele JJ, Jeuris W, Van Marck E . Inflammation in prostate biopsies of men without prostatic malignancy or clinical prostatitis: correlation with total serum PSA and PSA density. Eur Urol 2000; 37: 404–412.
De Marzo AM, Marchi VL, Epstein JI, Nelson WG . Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol 1999; 155: 1985–1992.
De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer 2007; 7: 256–269.
Sutcliffe S, Platz EA . Inflammation in the etiology of prostate cancer: an epidemiologic perspective. Urol Oncol 2007; 25: 242–249.
Onishi RM, Gaffen SL . Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 2010; 129: 311–321.
Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med 2009; 15: 1016–1022.
Chae WJ, Bothwell AL . IL-17F deficiency inhibits small intestinal tumorigenesis in ApcMin/+ mice. Biochem Biophys Res Commun 2011; 414: 31–36.
Chae WJ, Gibson TF, Zelterman D, Hao L, Henegariu O, Bothwell AL . Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc Natl Acad Sci USA 2010; 107: 5540–5544.
Hyun YS, Han DS, Lee AR, Eun CS, Youn JH, Kim HY . Role of IL-17A in the development of colitis-associated cancer. Carcinogenesis 2012; 33: 931–936.
Xiao M, Wang C, Zhang J, Li Z, Zhao X, Qin Z . IFNgamma promotes papilloma development by up-regulating Th17-associated inflammation. Cancer Res 2009; 69: 2010–2017.
Wang L, Yi T, Zhang W, Pardoll DM, Yu H . IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Res 2010; 70: 10112–10120.
Novitskiy SV, Pickup MW, Gorska AE, Owens P, Chytil A, Aakre M et al. TGF-beta receptor II loss promotes mammary carcinoma progression by Th17 dependent mechanisms. Cancer Discov 2011; 1: 430–441.
Zhang Q, Liu S, Ge D, Xue Y, Xiong Z, Abdel-Mageed AB et al. Interleukin-17 promotes formation and growth of prostate adenocarcinoma in mouse models. Cancer Res 2012; 72: 2589–2599.
Zhang Q, Liu S, Xiong Z, Wang AR, Myers L, Melamed J et al. Interleukin-17 promotes development of castration-resistant prostate cancer potentially through creating an immunotolerant and pro-angiogenic tumor microenvironment. Prostate 2014; 74: 869–879.
Chang SH, Mirabolfathinejad SG, Katta H, Cumpian AM, Gong L, Caetano MS et al. T helper 17 cells play a critical pathogenic role in lung cancer. Proc Natl Acad Sci USA 2014; 111: 5664–5669.
Xu B, Guenther JF, Pociask DA, Wang Y, Kolls JK, You Z et al. Promotion of lung tumor growth by interleukin-17. Am J Physiol Lung Cell Mol Physiol 2014; 307: L497–L508.
McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell 2014; 25: 621–637.
McAleer JP, Kolls JK . Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol Rev 2014; 260: 129–144.
Liu C, Qian W, Qian Y, Giltiay NV, Lu Y, Swaidani S et al. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci Signal 2009; 2: ra63.
Wilson CL, Matrisian LM . Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem Cell Biol 1996; 28: 123–136.
Pajouh MS, Nagle RB, Breathnach R, Finch JS, Brawer MK, Bowden GT . Expression of metalloproteinase genes in human prostate cancer. J Cancer Res Clin Oncol 1991; 117: 144–150.
Knox JD, Wolf C, McDaniel K, Clark V, Loriot M, Bowden GT et al. Matrilysin expression in human prostate carcinoma. Mol Carcinog 1996; 15: 57–63.
Radisky DC . Epithelial-mesenchymal transition. J Cell Sci 2005; 118: 4325–4326.
Thiery JP, Acloque H, Huang RY, Nieto MA . Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139: 871–890.
Gilles C, Polette M, Piette J, Birembaut P, Foidart JM . Epithelial-to-mesenchymal transition in HPV-33-transfected cervical keratinocytes is associated with increased invasiveness and expression of gelatinase A. Int J Cancer 1994; 59: 661–666.
Kang Y, Massague J . Epithelial-mesenchymal transitions: twist in development and metastasis. Cell 2004; 118: 277–279.
Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004; 117: 927–939.
Yang J, Weinberg RA . Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 2008; 14: 818–829.
Wilson CL, Heppner KJ, Labosky PA, Hogan BL, Matrisian LM . Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci USA 1997; 94: 1402–1407.
Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 2003; 4: 209–221.
Cunningham D, You Z . In vitro and in vivo model systems used in prostate cancer research. J Biol Methods 2015; 2: e17 1–14.
Nishizuka I, Ichikawa Y, Ishikawa T, Kamiyama M, Hasegawa S, Momiyama N et al. Matrilysin stimulates DNA synthesis of cultured vascular endothelial cells and induces angiogenesis in vivo. Cancer Lett 2001; 173: 175–182.
Huo N, Ichikawa Y, Kamiyama M, Ishikawa T, Hamaguchi Y, Hasegawa S et al. MMP-7 (matrilysin) accelerated growth of human umbilical vein endothelial cells. Cancer Lett 2002; 177: 95–100.
Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 2001; 114: 111–118.
Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ . Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol 1997; 139: 1861–1872.
Davies G, Jiang WG, Mason MD . Matrilysin mediates extracellular cleavage of E-cadherin from prostate cancer cells: a key mechanism in hepatocyte growth factor/scatter factor-induced cell-cell dissociation and in vitro invasion. Clin Cancer Res 2001; 7: 3289–3297.
Debelec-Butuner B, Alapinar C, Ertunc N, Gonen-Korkmaz C, Yorukoglu K, Korkmaz KS . TNFalpha-mediated loss of beta-catenin/E-cadherin association and subsequent increase in cell migration is partially restored by NKX3.1 expression in prostate cells. PLoS One 2014; 9: e109868.
Tsai JH, Yang J . Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev 2013; 27: 2192–2206.
Edman K, Furber M, Hemsley P, Johansson C, Pairaudeau G, Petersen J et al. The discovery of MMP7 inhibitors exploiting a novel selectivity trigger. ChemMedChem 2011; 6: 769–773.
Martin-Orozco N, Dong C . The IL-17/IL-23 axis of inflammation in cancer: friend or foe? Curr Opin Investig Drugs 2009; 10: 543–549.
Zou W, Restifo NP . T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol 2010; 10: 248–256.
Tartour E, Fossiez F, Joyeux I, Galinha A, Gey A, Claret E et al. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res 1999; 59: 3698–3704.
Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood 2003; 101: 2620–2627.
Hirahara N, Nio Y, Sasaki S, Minari Y, Takamura M, Iguchi C et al. Inoculation of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumor-specific immunity in mice. Oncology 2001; 61: 79–89.
Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood 2002; 99: 2114–2121.
Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H . IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med 2009; 206: 1457–1464.
Kryczek I, Wei S, Szeliga W, Vatan L, Zou W . Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood 2009; 114: 357–359.
Gu K, Li MM, Shen J, Liu F, Cao JY, Jin S et al. Interleukin-17-induced EMT promotes lung cancer cell migration and invasion via NF-kappaB/ZEB1 signal pathway. Am J Cancer Res 2015; 5: 1169–1179.
Wang L, Ma R, Kang Z, Zhang Y, Ding H, Guo W et al. Effect of IL-17A on the migration and invasion of NPC cells and related mechanisms. PLoS One 2014; 9: e108060.
Wu S, Lim KC, Huang J, Saidi RF, Sears CL . Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc Natl Acad Sci USA 1998; 95: 14979–14984.
Wu S, Morin PJ, Maouyo D, Sears CL . Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology 2003; 124: 392–400.
Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW et al. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J Cell Biol 2001; 152: 87–96.
You Z, Saims D, Chen S, Zhang Z, Guttridge DC, Guan K-l et al. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J Cell Biol 2002; 157: 429–440.
Easwaran V, Lee SH, Inge L, Guo L, Goldbeck C, Garrett E et al. beta-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res 2003; 63: 3145–3153.
Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res 2004; 64: 2270–2305.
Li Q, Lambrechts MJ, Zhang Q, Liu S, Ge D, Yin R et al. Glyphosate and AMPA inhibit cancer cell growth through inhibiting intracellular glycine synthesis. Drug Des Devel Ther 2013; 7: 635–643.
Chen C, Zhang Q, Liu S, Parajuli KR, Qu Y, Mei J et al. IL-17 and insulin/IGF1 enhance adhesion of prostate cancer cells to vascular endothelial cells through CD44-VCAM-1 interaction. Prostate 2015; 75: 883–895.
Liu S, Zhang Q, Chen C, Ge D, Qu Y, Chen R et al. Hyperinsulinemia enhances interleukin-17-induced inflammation to promote prostate cancer development in obese mice through inhibiting glycogen synthase kinase 3-mediated phosphorylation and degradation of interleukin-17 receptor. Oncotarget 2016; 7: 13651–13666.
Wang K, Singh D, Zeng Z, Coleman SJ, Huang Y, Savich GL et al. MapSplice: accurate mapping of RNA-seq reads for splice junction discovery. Nucleic Acids Res 2010; 38: e178.
Li B, Ruotti V, Stewart RM, Thomson JA, Dewey CN . RNA-Seq gene expression estimation with read mapping uncertainty. Bioinformatics 2010; 26: 493–500.
Acknowledgements
ZY was partially supported by the National Institutes of Health (R01CA174714 and P20GM103518), Department of Defense (W81XWH-14-1-0050, W81XWH-14-1-0149, W81XWH-14-1-0458 (PI: Feng Chen; Co-I: ZY), and W81XWH-15-1-0444), the Developmental Fund of Tulane Cancer Center (TCC), Louisiana Cancer Research Consortium (LCRC) Fund and Tulane’s Institute of Integrated Engineering for Health and Medicine (TI2EHM). WZ and KZ were partially supported by the National Institute on Minority Health and Health Disparities (5G12MD007595, PIs: Gene D’Amour and Guangdi Wang).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Zhang, Q., Liu, S., Parajuli, K. et al. Interleukin-17 promotes prostate cancer via MMP7-induced epithelial-to-mesenchymal transition. Oncogene 36, 687–699 (2017). https://doi.org/10.1038/onc.2016.240
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.240
This article is cited by
-
Blockade of C5aR1 resets M1 via gut microbiota-mediated PFKM stabilization in a TLR5-dependent manner
Cell Death & Disease (2024)
-
Litchi procyanidins inhibit colon cancer proliferation and metastasis by triggering gut-lung axis immunotherapy
Cell Death & Disease (2023)
-
Immunoproteasome inhibition prevents progression of castration-resistant prostate cancer
British Journal of Cancer (2023)
-
Microbiota as the unifying factor behind the hallmarks of cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
Relationship between Th17 cell proportion and IL-17/-18 levels and disease progression in patients with multiple myeloma
Molecular & Cellular Toxicology (2023)