Abstract
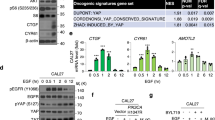
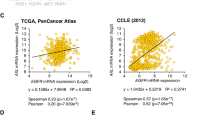
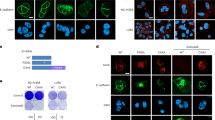
Atypical protein kinase Cι (PKCι) is an oncogene in lung and ovarian cancer. The PKCι gene PRKCI is targeted for frequent tumor-specific copy number gain (CNG) in both lung squamous cell carcinoma (LSCC) and ovarian serous carcinoma (OSC). We recently demonstrated that in LSCC cells PRKCI CNG functions to drive transformed growth and tumorigenicity by activating PKCι-dependent cell autonomous Hedgehog (Hh) signaling. Here, we assessed whether OSC cells harboring PRKCI CNG exhibit similar PKCι-dependent Hh signaling. Surprisingly, we find that whereas PKCι is required for the transformed growth of OSC cells harboring PRKCI CNG, these cells do not exhibit PKCι-dependent Hh signaling or Hh-dependent proliferation. Rather, transformed growth of OSC cells is regulated by PKCι-dependent nuclear localization of the oncogenic transcription factor, YAP1. Lentiviral shRNA-mediated knockdown (KD) of PKCι leads to decreased nuclear YAP1 and increased YAP1 binding to angiomotin (AMOT), which sequesters YAP1 in the cytoplasm. Biochemical analysis reveals that PKCι directly phosphorylates AMOT at a unique site, Thr750, whose phosphorylation inhibits YAP1 binding. Pharmacologic inhibition of PKCι decreases YAP1 nuclear localization and blocks OSC tumor growth in vitro and in vivo. Immunohistochemical analysis reveals a strong positive correlation between tumor PKCι expression and nuclear YAP1 in primary OSC tumor samples, indicating the clinical relevance of PKCι–YAP1 signaling. Our results uncover a novel PKCι–AMOT–YAP1 signaling axis that promotes OSC tumor growth, and provide a rationale for therapeutic targeting of this pathway for treatment of OSC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A . Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29.
Regala RP, Weems C, Jamieson L, Khoor A, Edell ES, Lohse CM et al. Atypical protein kinase C iota is an oncogene in human non-small cell lung cancer. Cancer Res 2005; 65: 8905–8911.
Justilien V, Walsh MP, Ali SA, Thompson EA, Murray NR, Fields AP . The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer Cell 2014; 25: 139–151.
Wang Y, Hill KS, Fields AP . PKCiota maintains a tumor-initiating cell phenotype that is required for ovarian tumorigenesis. Mol Cancer Res 2013; 11: 1624–1635.
Ciriello G, Miller ML, Aksoy BA, Senbabaoglu Y, Schultz N, Sander C . Emerging landscape of oncogenic signatures across human cancers. Nat Genet 2013; 45: 1127–1133.
Eder AM, Sui X, Rosen DG, Nolden LK, Cheng KW, Lahad JP et al. Atypical PKCiota contributes to poor prognosis through loss of apical-basal polarity and cyclin E overexpression in ovarian cancer. Proc Natl Acad Sci USA 2005; 102: 12519–12524.
Zhang L, Huang J, Yang N, Liang S, Barchetti A, Giannakakis A et al. Integrative genomic analysis of protein kinase C (PKC) family identifies PKCiota as a biomarker and potential oncogene in ovarian carcinoma. Cancer Res 2006; 66: 4627–4635.
Ali SA, Justilien V, Jamieson L, Murray NR, Fields AP . Protein kinase C iota drives a NOTCH3-dependent stem-like phenotype in mutant KRAS lung adenocarcinoma. Cancer Cell 2016; 29: 367–378.
Wang Y, Hill KS, Fields AP . Protein kinase C iota maintains a tumor-initiating cell phenotype that is required for ovarian tumorigenesis. Mol Cancer Res 2013; 11: 1624–1635.
Regala RP, Weems C, Jamieson L, Copland JA, Thompson EA, Fields AP . Atypical protein kinase C iota plays a critical role in human lung cancer cell growth and tumorigenicity. J Biol Chem 2005; 280: 31109–31115.
Murray NR, Kalari KR, Fields AP . Protein kinase C iota expression and oncogenic signaling mechanisms in cancer. J Cell Physiol 2011; 226: 879–887.
Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012; 483: 603–607.
Domcke S, Sinha R, Levine DA, Sander C, Schultz N . Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun 2013; 4: 2126.
Hall CA, Wang R, Miao J, Oliva E, Shen X, Wheeler T et al. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res 2010; 70: 8517–8525.
Zhang X, George J, Deb S, Degoutin JL, Takano EA, Fox SB et al. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene 2011; 30: 2810–2822.
Archibald A, Al-Masri M, Liew-Spilger A, McCaffrey L . Atypical protein kinase C induces cell transformation by disrupting Hippo/Yap signaling. Mol Biol Cell 2015; 26: 3578–3595.
Hsu YL, Hung JY, Chou SH, Huang MS, Tsai MJ, Lin YS et al. Angiomotin decreases lung cancer progression by sequestering oncogenic YAP/TAZ and decreasing Cyr61 expression. Oncogene 2015; 34: 4056–4068.
Jeong GO, Shin SH, Seo EJ, Kwon YW, Heo SC, Kim KH et al. TAZ mediates lysophosphatidic acid-induced migration and proliferation of epithelial ovarian cancer cells. Cell Physiol Biochem 2013; 32: 253–263.
Rho SB, Woo JS, Chun T, Park SY . Cysteine-rich 61 (CYR61) inhibits cisplatin-induced apoptosis in ovarian carcinoma cells. Biotechnol Lett 2009; 31: 23–28.
Shen H, Cai M, Zhao S, Wang H, Li M, Yao S et al. CYR61 overexpression associated with the development and poor prognosis of ovarian carcinoma. Med Oncol 2014; 31: 117.
Moleirinho S, Guerrant W, Kissil JL . The angiomotins—from discovery to function. FEBS Lett 2014; 588: 2693–2703.
Llado V, Nakanishi Y, Duran A, Reina-Campos M, Shelton PM, Linares JF et al. Repression of intestinal stem cell function and tumorigenesis through direct phosphorylation of beta-catenin and Yap by PKCzeta. Cell Rep 2015, e-pub ahead of print 4 February 2015 doi:10.1016/j.celrep.2015.01.007.
Chan SW, Lim CJ, Chong YF, Pobbati AV, Huang C, Hong W . Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem 2011; 286: 7018–7026.
Yi C, Shen Z, Stemmer-Rachamimov A, Dawany N, Troutman S, Showe LC et al. The p130 isoform of angiomotin is required for Yap-mediated hepatic epithelial cell proliferation and tumorigenesis. Sci Signal 2013; 6: ra77.
Butler AM, Scotti Buzhardt ML, Erdogan E, Li S, Inman KS, Fields AP et al. A small molecule inhibitor of atypical protein kinase C signaling inhibits pancreatic cancer cell transformed growth and invasion. Oncotarget 2015; 6: 15297–15310.
Korkmaz T, Seber S, Basaran G . Review of the current role of targeted therapies as maintenance therapies in first and second line treatment of epithelial ovarian cancer; in the light of completed trials. Crit Rev Oncol Hematol 2015; 98: 180–188.
Martin LP, Schilder RJ . Management of recurrent ovarian carcinoma: current status and future directions. Semin Oncol 2009; 36: 112–125.
Petrucelli N, Daly MB, Feldman GL . Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2. Genet Med 2010; 12: 245–259.
Sugita M, Tanaka N, Davidson S, Sekiya S, Varella-Garcia M, West J et al. Molecular definition of a small amplification domain within 3q26 in tumors of cervix, ovary, and lung. Cancer Genet Cytogenet 2000; 117: 9–18.
Sonoda G, Palazzo J, du Manoir S, Godwin AK, Feder M, Yakushiji M et al. Comparative genomic hybridization detects frequent overrepresentation of chromosomal material from 3q26, 8q24, and 20q13 in human ovarian carcinomas. Genes Chromosomes Cancer 1997; 20: 320–328.
Arnold N, Hagele L, Walz L, Schempp W, Pfisterer J, Bauknecht T et al. Overrepresentation of 3q and 8q material and loss of 18q material are recurrent findings in advanced human ovarian cancer. Genes Chromosomes Cancer 1996; 16: 46–54.
Haverty PM, Hon LS, Kaminker JS, Chant J, Zhang Z . High-resolution analysis of copy number alterations and associated expression changes in ovarian tumors. BMC Med Genomics 2009; 2: 21.
Fields AP, Regala RP . Protein kinase C iota: human oncogene, prognostic marker and therapeutic target. Pharmacol Res 2007; 55: 487–497.
Parker PJ, Justilien V, Riou P, Linch M, Fields AP . Atypical protein kinase Ciota as a human oncogene and therapeutic target. Biochem Pharmacol 2014; 88: 1–11.
Fields AP, Murray NR . Protein kinase C isozymes as therapeutic targets for treatment of human cancers. Adv Enzyme Regul 2008; 48: 166–178.
Fields AP, Gustafson WC . Protein kinase C in disease: cancer. Methods Mol Biol 2003; 233: 519–537.
Jatoi A, Radecki Breitkopf C, Foster NR, Block MS, Grudem M, Wahner Hendrickson A et al. A Mixed-Methods Feasibility Trial of protein kinase C iota inhibition with Auranofin in asymptomatic ovarian cancer patients. Oncology 2014; 88: 208–213.
Algeciras-Schimnich A, Milosevic D, McIver B, Flynn H, Reddi HV, Eberhardt NL et al. Evaluation of the PAX8/PPARG translocation in follicular thyroid cancer with a 4-color reverse-transcription PCR assay and automated high-resolution fragment analysis. Clin Chem 2010; 56: 391–398.
Frederick LA, Matthews JA, Jamieson L, Justilien V, Thompson EA, Radisky DC et al. Matrix metalloproteinase-10 is a critical effector of protein kinase Ciota-Par6alpha-mediated lung cancer. Oncogene 2008; 27: 4841–4853.
Justilien V, Jameison L, Der CJ, Rossman KL, Fields AP . Oncogenic activity of Ect2 is regulated through protein kinase C iota-mediated phosphorylation. J Biol Chem 2011; 286: 8149–8157.
Acknowledgements
We thank Dr Kim Kalli and the Mayo Clinic Ovarian SPORE Tissue shared resource for ovarian cancer tissue microarrays, The Mayo Clinic Cytogenetics Core for FISH analysis, Ms Brandy Edenfield for tissue processing and immunohistochemical staining, and Kayla Lewis, Capella Weems and Dr Christelle Colin Cassin for technical assistance. This research was supported by the Mayo Clinic Specialized Program in Research Excellence (SPORE) grant CA136393 from the National Institutes of Health (APF is a Project Leader on this grant), a post-doctoral fellowship award from the Ovarian Cancer Research Fund to YW, National Cancer Institute grants R21 CA204938-01 (to VJ) and R01 CA081436-18 (to APF), and the George Haub Family Career Development Award (to VJ). APF is the Monica Flynn Jacoby Professor of Cancer Research, an endowment fund that provides partial support for his research program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, Y., Justilien, V., Brennan, K. et al. PKCι regulates nuclear YAP1 localization and ovarian cancer tumorigenesis. Oncogene 36, 534–545 (2017). https://doi.org/10.1038/onc.2016.224
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.224
This article is cited by
-
The role of Motin family proteins in tumorigenesis—an update
Oncogene (2023)
-
Amplified therapeutic targets in high-grade serous ovarian carcinoma – a review of the literature with quantitative appraisal
Cancer Gene Therapy (2023)
-
AMOT suppresses tumor progression via regulating DNA damage response signaling in diffuse large B-cell lymphoma
Cancer Gene Therapy (2021)
-
Angiomotin-p130 inhibits vasculogenic mimicry formation of small cell lung cancer independently of Smad2/3 signal pathway
Journal of Bioenergetics and Biomembranes (2021)
-
CLIC1 knockout inhibits invasion and migration of gastric cancer by upregulating AMOT-p130 expression
Clinical and Translational Oncology (2021)