Abstract
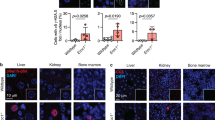
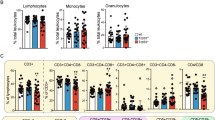
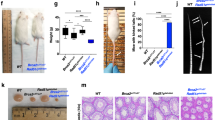
Mdm2, the principal negative regulator of p53, is critical for survival, a fact clearly demonstrated by the p53-dependent death of germline or conditional mice following deletion of Mdm2. On the other hand, Mdm2 hypomorphic (Mdm2Puro/Δ7−12) or heterozygous (Mdm2+/−) mice that express either 30 or 50% of normal Mdm2 levels, respectively, are viable but present distinct phenotypes because of increased p53 activity. Mdm2 levels are also transcriptionally regulated by p53. We evaluated the significance of this reciprocal relationship in a new hypomorphic mouse model inheriting an aberrant Mdm2 allele with insertion of the neomycin cassette and deletion of 184-bp sequence in intron 3. These mice also carry mutations in the Mdm2 P2-promoter and thus express suboptimal levels of Mdm2 entirely encoded from the P1-promoter. Resulting mice exhibit abnormalities in skin pigmentation and reproductive tissue architecture, and are subfertile. Notably, all these phenotypes are rescued on a p53-null background. Furthermore, these phenotypes depend on distinct p53 downstream activities as genetic ablation of the pro-apoptotic gene Puma reverts the reproductive abnormalities but not skin hyperpigmentation, whereas deletion of cell cycle arrest gene p21 does not rescue either phenotype. Moreover, p53-mediated upregulation of Kitl influences skin pigmentation. Altogether, these data emphasize tissue-specific p53 activities that regulate cell fate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Jones SN, Roe AE, Donehower LA, Bradley A . Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 1995; 378: 206–208.
Montes de Oca Luna R, Wagner DS, Lozano G . Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 1995; 378: 203–206.
Francoz S, Froment P, Bogaerts S, De Clercq S, Maetens M, Doumont G et al. Mdm4 and Mdm2 cooperate to inhibit p53 activity in proliferating and quiescent cells in vivo. Proc Natl Acad Sci USA 2006; 103: 3232–3237.
Grier JD, Xiong S, Elizondo-Fraire AC, Parant JM, Lozano G . Tissue-specific differences of p53 inhibition by Mdm2 and Mdm4. Mol Cell Biol 2006; 26: 192–198.
Xiong S, Van Pelt CS, Elizondo-Fraire AC, Liu G, Lozano G . Synergistic roles of Mdm2 and Mdm4 for p53 inhibition in central nervous system development. Proc Natl Acad Sci USA 2006; 103: 3226–3231.
Zhang Y, Xiong S, Li Q, Hu S, Tashakori M, Van Pelt C et al. Tissue-specific and age-dependent effects of global Mdm2 loss. J Pathol 2014; 233: 380–391.
Terzian T, Wang Y, Van Pelt CS, Box NF, Travis EL, Lozano G . Haploinsufficiency of Mdm2 and Mdm4 in tumorigenesis and development. Mol Cell Biol 2007; 27: 5479–5485.
Mendrysa SM, McElwee MK, Michalowski J, O'Leary KA, Young KM, Perry ME . Mdm2 Is critical for inhibition of p53 during lymphopoiesis and the response to ionizing irradiation. Mol Cell Biol 2003; 23: 462–472.
Barak Y, Juven T, Haffner R, Oren M . Mdm2 expression is induced by wild type p53 activity. EMBO J 1993; 12: 461–468.
Barak Y, Lupo A, Zauberman A, Juven T, Aloni-Grinstein R, Gottlieb E et al. Targets for transcriptional activation by wild-type p53: endogenous retroviral LTR, immunoglobulin-like promoter, and an internal promoter of the mdm2 gene. Cold Spring Harb Symp Quant Biol 1994; 59: 225–235.
Wu X, Bayle JH, Olson D, Levine AJ . The p53-mdm-2 autoregulatory feedback loop. Genes Dev 1993; 7: 1126–1132.
Mendrysa SM, Perry ME . The p53 tumor suppressor protein does not regulate expression of its own inhibitor, MDM2, except under conditions of stress. Mol Cell Biol 2000; 20: 2023–2030.
Pant V, Xiong S, Jackson JG, Post SM, Abbas HA, Quintas-Cardama A et al. The p53-Mdm2 feedback loop protects against DNA damage by inhibiting p53 activity but is dispensable for p53 stability, development, and longevity. Genes Dev 2013; 27: 1857–1867.
Haupt Y, Maya R, Kazaz A, Oren M . Mdm2 promotes the rapid degradation of p53. Nature 1997; 387: 296–299.
Honda R, Tanaka H, Yasuda H . Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett 1997; 420: 25–27.
McGowan KA, Li JZ, Park CY, Beaudry V, Tabor HK, Sabnis AJ et al. Ribosomal mutations cause p53-mediated dark skin and pleiotropic effects. Nat Genet 2008; 40: 963–970.
McGowan KA, Pang WW, Bhardwaj R, Perez MG, Pluvinage JV, Glader BE et al. Reduced ribosomal protein gene dosage and p53 activation in low-risk myelodysplastic syndrome. Blood 2011; 118: 3622–3633.
Terzian T, Dumble M, Arbab F, Thaller C, Donehower LA, Lozano G et al. Rpl27a mutation in the sooty foot ataxia mouse phenocopies high p53 mouse models. J Pathol 2011; 224: 540–552.
Itahana K, Mao H, Jin A, Itahana Y, Clegg HV, Lindstrom MS et al. Targeted inactivation of Mdm2 RING finger E3 ubiquitin ligase activity in the mouse reveals mechanistic insights into p53 regulation. Cancer Cell 2007; 12: 355–366.
Pant V, Lozano G . Dissecting the p53-Mdm2 feedback loop in vivo: uncoupling the role in p53 stability and activity. Oncotarget 2014; 5: 1149–1156.
Liu G, Terzian T, Xiong S, Van Pelt CS, Audiffred A, Box NF et al. The p53-Mdm2 network in progenitor cell expansion during mouse postnatal development. J Pathol 2007; 213: 360–368.
Zeron-Medina J, Wang X, Repapi E, Campbell MR, Su D, Castro-Giner F et al. A polymorphic p53 response element in KIT ligand influences cancer risk and has undergone natural selection. Cell 2013; 155: 410–422.
Vanover JC, Spry ML, Hamilton L, Wakamatsu K, Ito S, D'Orazio JA . Stem cell factor rescues tyrosinase expression and pigmentation in discreet anatomic locations in albino mice. Pigment Cell Melanoma Res 2009; 22: 827–838.
Kunisada T, Yoshida H, Yamazaki H, Miyamoto A, Hemmi H, Nishimura E et al. Transgene expression of steel factor in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors. Development 1998; 125: 2915–2923.
Okura M, Maeda H, Nishikawa S, Mizoguchi M . Effects of monoclonal anti-c-kit antibody (ACK2) on melanocytes in newborn mice. J Invest Dermatol 1995; 105: 322–328.
Perry ME . The regulation of the p53-mediated stress response by MDM2 and MDM4. Cold Spring Harb Perspect Biol 2010; 2: a000968.
Steingrimsson E, Copeland NG, Jenkins NA . Mouse coat color mutations: from fancy mice to functional genomics. Dev Dyn 2006; 235: 2401–2411.
Beumer TL, Roepers-Gajadien HL, Gademan IS, van Buul PP, Gil-Gomez G, Rutgers DH et al. The role of the tumor suppressor p53 in spermatogenesis. Cell Death Differ 1998; 5: 669–677.
Almon E, Goldfinger N, Kapon A, Schwartz D, Levine AJ, Rotter V . Testicular tissue-specific expression of the p53 suppressor gene. Dev Biol 1993; 156: 107–116.
Sullivan JM, Jeffords LB, Lee CL, Rodrigues R, Ma Y, Kirsch DG . p21 protects 'Super p53' mice from the radiation-induced gastrointestinal syndrome. Radiat Res 2012; 177: 307–310.
Post SM, Quintas-Cardama A, Pant V, Iwakuma T, Hamir A, Jackson JG et al. A high-frequency regulatory polymorphism in the p53 pathway accelerates tumor development. Cancer Cell 2010; 18: 220–230.
Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 2003; 4: 321–328.
Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol 1994; 4: 1–7.
Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ . Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 1995; 377: 552–557.
Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L et al. Restoration of p53 function leads to tumour regression in vivo. Nature 2007; 445: 661–665.
Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A . Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev 2000; 14: 994–1004.
Shetty G, Wilson G, Huhtaniemi I, Boettger-Tong H, Meistrich ML . Testosterone inhibits spermatogonial differentiation in juvenile spermatogonial depletion mice. Endocrinology 2001; 142: 2789–2795.
Acknowledgements
Mdm2PND/PND mice were made by the GEM Facility at the MDACC funded by an NCI Cancer Center Support Grant (CA16672). The studies were supported by NIH grant CA47296 to GL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Pant, V., Xiong, S., Chau, G. et al. Distinct downstream targets manifest p53-dependent pathologies in mice. Oncogene 35, 5713–5721 (2016). https://doi.org/10.1038/onc.2016.111
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.111
This article is cited by
-
Tissue specificity and spatio-temporal dynamics of the p53 transcriptional program
Cell Death & Differentiation (2023)
-
p53-dependent induction of P2X7 on hematopoietic stem and progenitor cells regulates hematopoietic response to genotoxic stress
Cell Death & Disease (2021)
-
Relevance of the p53–MDM2 axis to aging
Cell Death & Differentiation (2018)
-
Curcumin suppresses gastric tumor cell growth via ROS-mediated DNA polymerase γ depletion disrupting cellular bioenergetics
Journal of Experimental & Clinical Cancer Research (2017)