Abstract
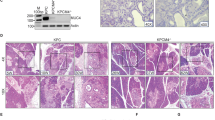
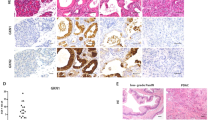
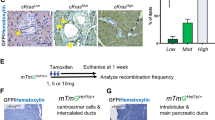
Cadherin subtype switching from E-cadherin to N-cadherin is associated with the epithelial-to-mesenchymal transition (EMT), a process required for invasion and dissemination of carcinoma cells. We found that N-cadherin is expressed in human and mouse pancreatic intraepithelial neoplasia (PanIN), suggesting that N-cadherin may also have a role in early-stage pancreatic cancer. To investigate the role of N-cadherin in mouse PanIN (mPanIN), we simultaneously activated oncogenic K-rasG12D and deleted the N-cadherin (Cdh2) gene in the murine pancreas. Genetic ablation of N-cadherin (N-cad KO) caused hyperproliferation, accelerated mPanIN progression, and early tumor development in K-rasG12D mice. Decreased E-cadherin and redistribution of β-catenin accompanied the loss of N-cadherin in pancreatic ductal epithelial cells (PDEC). Nuclear accumulation of β-catenin and its transcription co-activator Tcf4 led to activation of Wnt/β-catenin target genes. Unexpectedly, loss of N-cadherin in the K-rasG12D model resulted in increased mPanIN progression and tumor incidence. These in vivo results demonstrate for the first time that N-cadherin functions as a growth suppressor in the context of oncogenic K-ras.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ryan DP, Hong TS, Bardeesy N . Pancreatic adenocarcinoma. N Engl J Med 2014; 371: 1039–1049.
Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 2010; 467: 1109–1113.
Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008; 321: 1801–1806.
van Roy F . Beyond E-cadherin: roles of other cadherin superfamily members in cancer. Nat Rev Cancer 2014; 14: 121–134.
Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG . Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res 2007; 13: 4769–4776.
Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR . Cadherin switching. J Cell Sci 2008; 121: 727–735.
Maeda M, Johnson KR, Wheelock MJ . Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci 2005; 118: 873–887.
Qian X, Anzovino A, Kim S, Suyama K, Yao J, Hulit J et al. N-cadherin/FGFR promotes metastasis through epithelial-to-mesenchymal transition and stem/progenitor cell-like properties. Oncogene 2014; 33: 3411–3421.
Shintani Y, Hollingsworth MA, Wheelock MJ, Johnson KR . Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res 2006; 66: 11745–53.
Su Y, Li J, Witkiewicz AK, Brennan D, Neill T, Talarico J et al. N-cadherin haploinsufficiency increases survival in a mouse model of pancreatic cancer. Oncogene 2012; 31: 4484–4489.
Al-Aynati MM, Radulovich N, Riddell RH, Tsao MS . Epithelial-cadherin and beta-catenin expression changes in pancreatic intraepithelial neoplasia. Clin Cancer Res 2004; 10: 1235–1240.
Pasca di Magliano M, Biankin AV, Heiser PW, Cano DA, Gutierrez PJ, Deramaudt T et al. Common activation of canonical Wnt signaling in pancreatic adenocarcinoma. PLoS One 2007; 2: e1155.
Nakajima S, Doi R, Toyoda E, Tsuji S, Wada M, Koizumi M et al. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin Cancer Res 2004; 10: 4125–4133.
Zhang Y, JPt Morris, Yan W, Schofield HK, Gurney A, Simeone DM et al. Canonical wnt signaling is required for pancreatic carcinogenesis. Cancer Res 2013; 73: 4909–4922.
Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003; 4: 437–450.
Luo Y, Kostetskii I, Radice GL . N-cadherin is not essential for limb mesenchymal chondrogenesis. Dev Dyn 2005; 232: 336–344.
Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005; 7: 469–483.
Kostetskii I, Li J, Xiong Y, Zhou R, Ferrari VA, Patel VV et al. Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ Res 2005; 96: 346–354.
Johansson JK, Voss U, Kesavan G, Kostetskii I, Wierup N, Radice GL et al. N-cadherin is dispensable for pancreas development but required for beta-cell granule turnover. Genesis 2010; 48: 374–381.
Hruban RH, Adsay NV, Albores-Saavedra J, Anver MR, Biankin AV, Boivin GP et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res 2006; 66: 95–106.
Gottardi CJ, Wong E, Gumbiner BM . E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. J Cell Biol 2001; 153: 1049–1060.
Schreiber FS, Deramaudt TB, Brunner TB, Boretti MI, Gooch KJ, Stoffers DA et al. Successful growth and characterization of mouse pancreatic ductal cells: functional properties of the Ki-RAS(G12V) oncogene. Gastroenterology 2004; 127: 250–260.
McClatchey AI, Yap AS . Contact inhibition (of proliferation) redux. Curr Opin Cell Biol 2012; 24: 685–694.
Agbunag C, Bar-Sagi D . Oncogenic K-ras drives cell cycle progression and phenotypic conversion of primary pancreatic duct epithelial cells. Cancer Res 2004; 64: 5659–5663.
Deramaudt TB, Takaoka M, Upadhyay R, Bowser MJ, Porter J, Lee A et al. N-cadherin and keratinocyte growth factor receptor mediate the functional interplay between Ki-RASG12V and p53V143A in promoting pancreatic cell migration, invasion, and tissue architecture disruption. Mol Cell Biol 2006; 26: 4185–4200.
Levenberg S, Yarden A, Kam Z, Geiger B . p27 is involved in N-cadherin-mediated contact inhibition of cell growth and S-phase entry. Oncogene 1999; 18: 869–876.
Charrasse S, Meriane M, Comunale F, Blangy A, Gauthier-Rouviere C . N-cadherin-dependent cell-cell contact regulates Rho GTPases and beta-catenin localization in mouse C2C12 myoblasts. J Cell Biol 2002; 158: 953–965.
Gavard J, Marthiens V, Monnet C, Lambert M, Mege RM . N-cadherin activation substitutes for the cell contact control in cell cycle arrest and myogenic differentiation: involvement of p120 and beta-catenin. J Biol Chem 2004; 279: 36795–36802.
Li J, Gao E, Vite A, Yi R, Gomez L, Goossens S et al. Alpha-catenins control cardiomyocyte proliferation by regulating Yap activity. Circ Res 2015; 116: 70–79.
Mui KL, Bae YH, Gao L, Liu SL, Xu T, Radice GL et al. N-cadherin induction by ECM stiffness and FAK overrides the spreading requirement for proliferation of vascular smooth muscle cells. Cell Rep 2015; 10: 1477–1486.
Gumbiner BM, Kim NG . The Hippo-YAP signaling pathway and contact inhibition of growth. J Cell Sci 2014; 127: 709–717.
Kim NG, Koh E, Chen X, Gumbiner BM . E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci USA 2011; 108: 11930–11935.
Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ et al. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 2012; 151: 1457–1473.
Acknowledgements
We are grateful to Frans van Roy, Anil Rustgi and Jennifer Wilson for comments. We thank David Tuveson for the LSL-K-rasG12D mice, Andrew Lowy for the Pdx1/Cre mice and Michael Goggins for the PanIN microarrays. We are grateful to Mathew Thakur for assistance with PET imaging, and Han Du, Craig Riley and David Kurz for technical assistance. Research in this study includes work carried out by the Jefferson Kimmel Cancer Center Small Animal Imaging Facility, which is supported in part by NCI Cancer Center Support Grant P30 CA56036. This work was supported by NIH R21 CA176097 (GR). This study was also supported by the SPORE grant CA62924 (RH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Su, Y., Li, J., Shi, C. et al. N-cadherin functions as a growth suppressor in a model of K-ras-induced PanIN. Oncogene 35, 3335–3341 (2016). https://doi.org/10.1038/onc.2015.382
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.382
This article is cited by
-
Distribution of E- and N-cadherin in subgroups of non-functioning pituitary neuroendocrine tumours
Endocrine (2022)
-
Fibrinogen-like protein 1 (FGL1): the next immune checkpoint target
Journal of Hematology & Oncology (2021)
-
Targeting TRAF3IP2, Compared to Rab27, is More Effective in Suppressing the Development and Metastasis of Breast Cancer
Scientific Reports (2020)
-
N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer
BMC Cancer (2018)
-
Cadherin-1 and cadherin-3 cooperation determines the aggressiveness of pancreatic ductal adenocarcinoma
British Journal of Cancer (2018)