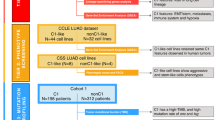
Abstract
While mutations in the KRAS oncogene are among the most prevalent in human cancer, there are few successful treatments to target these tumors. It is also likely that heterogeneity in KRAS-mutant tumor biology significantly contributes to the response to therapy. We hypothesized that the presence of commonly co-occurring mutations in STK11 and TP53 tumor suppressors may represent a significant source of heterogeneity in KRAS-mutant tumors. To address this, we utilized a large cohort of resected tumors from 442 lung adenocarcinoma patients with data including annotation of prevalent driver mutations (KRAS and EGFR) and tumor suppressor mutations (STK11 and TP53), microarray-based gene expression and clinical covariates, including overall survival (OS). Specifically, we determined impact of STK11 and TP53 mutations on a new KRAS mutation-associated gene expression signature as well as previously defined signatures of tumor cell proliferation and immune surveillance responses. Interestingly, STK11, but not TP53 mutations, were associated with highly elevated expression of KRAS mutation-associated genes. Mutations in TP53 and STK11 also impacted tumor biology regardless of KRAS status, with TP53 strongly associated with enhanced proliferation and STK11 with suppression of immune surveillance. These findings illustrate the remarkably distinct ways through which tumor suppressor mutations may contribute to heterogeneity in KRAS-mutant tumor biology. In addition, these studies point to novel associations between gene mutations and immune surveillance that could impact the response to immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Siegel R, Naishadham D, Jemal A . Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11–30.
Pao W, Girard N . New driver mutations in non-small-cell lung cancer. Lancet Oncol 2011; 12: 175–180.
Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 2008; 455: 1069–1075.
Weir BA, Woo MS, Getz G, Perner S, Ding L, Beroukhim R et al. Characterizing the cancer genome in lung adenocarcinoma. Nature 2007; 450: 893–898.
Cox AD, Der CJ . Ras history: the saga continues. Small GTPases 2010; 1: 2–27.
Mitin N, Rossman KL, Der CJ . Signaling interplay in Ras superfamily function. Curr Biol 2005; 15: R563–R574.
Roberts PJ, Der CJ . Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007; 26: 3291–3310.
Bang D, Wilson W, Ryan M, Yeh JJ, Baldwin AS . GSK-3alpha promotes oncogenic KRAS function in pancreatic cancer via TAK1-TAB stabilization and regulation of noncanonical NF-kappaB. Cancer Discov 2013; 3: 690–703.
Baker NM, Yee Chow H, Chernoff J, Der CJ . Molecular pathways: targeting RAC-p21-activated serine-threonine kinase signaling in RAS-driven cancers. Clin Cancer Res 2014; 20: 4740–4746.
Kim HS, Mendiratta S, Kim J, Pecot CV, Larsen JE, Zubovych I et al. Systematic identification of molecular subtype-selective vulnerabilities in non-small-cell lung cancer. Cell 2013; 155: 552–566.
Sos ML, Michel K, Zander T, Weiss J, Frommolt P, Peifer M et al. Predicting drug susceptibility of non-small cell lung cancers based on genetic lesions. J Clin Invest 2009; 119: 1727–1740.
Gibbons DL, Byers LA, Kurie JM . Smoking, p53 mutation, and lung cancer. Mol Cancer Res 2014; 12: 3–13.
Shackelford DB, Shaw RJ . The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer 2009; 9: 563–575.
Sanchez-Cespedes M, Parrella P, Esteller M, Nomoto S, Trink B, Engles JM et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res 2002; 62: 3659–3662.
Shah U, Sharpless NE, Hayes DN . LKB1 and lung cancer: more than the usual suspects. Cancer Res 2008; 68: 3562–3565.
Sanchez-Cespedes M . The role of LKB1 in lung cancer. Fam Cancer 2011; 10: 447–453.
Gao Y, Ge G, Ji H . LKB1 in lung cancerigenesis: a serine/threonine kinase as tumor suppressor. Protein Cell 2011; 2: 99–107.
Matsumoto S, Iwakawa R, Takahashi K, Kohno T, Nakanishi Y, Matsuno Y et al. Prevalence and specificity of LKB1 genetic alterations in lung cancers. Oncogene 2007; 26: 5911–5918.
Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P et al. LKB1 modulates lung cancer differentiation and metastasis. Nature 2007; 448: 807–810.
O'Neill GM, Seo S, Serebriiskii IG, Lessin SR, Golemis EA . A new central scaffold for metastasis: parsing HEF1/Cas-L/NEDD9. Cancer Res 2007; 67: 8975–8979.
Gao Y, Xiao Q, Ma H, Li L, Liu J, Feng Y et al. LKB1 inhibits lung cancer progression through lysyl oxidase and extracellular matrix remodeling. Proc Natl Acad Sci USA 2010; 107: 18892–18897.
Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 2006; 439: 353–357.
Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N et al. A gene expression signature associated with ‘K-Ras addiction’ reveals regulators of EMT and tumor cell survival. Cancer Cell 2009; 15: 489–500.
Loboda A, Nebozhyn M, Klinghoffer R, Frazier J, Chastain M, Arthur W et al. A gene expression signature of RAS pathway dependence predicts response to PI3K and RAS pathway inhibitors and expands the population of RAS pathway activated tumors. BMC Med Genomics 2010; 3: 26.
Chen DT, Hsu YL, Fulp WJ, Coppola D, Haura EB, Yeatman TJ et al. Prognostic and predictive value of a malignancy-risk gene signature in early-stage non-small cell lung cancer. J Natl Cancer Inst 2011; 103: 1859–1870.
Chen DT, Nasir A, Culhane A, Venkataramu C, Fulp W, Rubio R et al. Proliferative genes dominate malignancy-risk gene signature in histologically-normal breast tissue. Breast Cancer Res Treat 2010; 119: 335–346.
Dunn GP, Koebel CM, Schreiber RD . Interferons, immunity and cancer immunoediting. Nat Rev Immunol 2006; 6: 836–848.
Schreiber RD, Old LJ, Smyth MJ . Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331: 1565–1570.
Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001; 410: 1107–1111.
Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348: 203–213.
Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G . Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res 2011; 71: 5601–5605.
Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353: 2654–2666.
Yu H, Pardoll D, Jove R . STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009; 9: 798–809.
Yu H, Kortylewski M, Pardoll D . Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 2007; 7: 41–51.
Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ . Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011; 29: 235–271.
Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother 2012; 61: 1019–1031.
Ulloa-Montoya F, Louahed J, Dizier B, Gruselle O, Spiessens B, Lehmann FF et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol 2013; 31: 2388–2395.
Gajewski TF, Schreiber H, Fu YX . Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013; 14: 1014–1022.
Fuertes MB, Woo SR, Burnett B, Fu YX, Gajewski TF . Type I interferon response and innate immune sensing of cancer. Trends Immunol 2013; 34: 67–73.
Gajewski TF, Schumacher T . Cancer immunotherapy. Curr Opin Immunol 2013; 25: 259–260.
Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol 2013; 25: 268–276.
Hopewell EL, Zhao W, Fulp WJ, Bronk CC, Lopez AS, Massengill M et al. Lung tumor NF-kappaB signaling promotes T cell-mediated immune surveillance. J Clin Invest 2013; 123: 2509–2522.
Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res 2011; 39 (Database issue): D945–D950.
Meng D, Yuan M, Li X, Chen L, Yang J, Zhao X et al. Prognostic value of K-RAS mutations in patients with non-small cell lung cancer: a systematic review with meta-analysis. Lung Cancer 2013; 81: 1–10.
Kaufman JM, Amann JM, Park K, Arasada RR, Li H, Shyr Y et al. LKB1 Loss induces characteristic patterns of gene expression in human tumors associated with NRF2 activation and attenuation of PI3K-AKT. J Thorac Oncol 2014; 9: 794–804.
Carretero J, Shimamura T, Rikova K, Jackson AL, Wilkerson MD, Borgman CL et al. Integrative genomic and proteomic analyses identify targets for Lkb1-deficient metastatic lung tumors. Cancer Cell 2010; 17: 547–559.
Acknowledgements
This work was supported by a National Institutes of Health SPORE Grant (P50 CA119997). We would also like to thank Mr Andrew ‘Ross’ Myers, Anastasia Belock, Mercedes Rodriguez, Edward T Chwieseni, Marek Wloch, Hiba Gohar, and Moffitt’s Tissue Core facility, Moffitt’s Cancer Registry (Director: Karen A Coyne), Research Information Technology (IT) group, and the Data Management and Integration Technology (DMIT) group. Total Cancer Care® is enabled, in part, by the generous support of the DeBartolo Family, and we thank the many patients who provided data and tissue to the Total Cancer Care Consortium. Our study also received valuable assistance from the following Core Facilities at the Moffitt Cancer Center: Biostatistics and Cancer Informatics, Tissue, and Molecular Genomics. This work has been supported in part by a Cancer Center Support Grant (CCSG grant P30-CA76292) at the H. Lee Moffitt Cancer Center and Research Institute, a National Cancer Institute-designated Comprehensive Cancer Center.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Schabath, M., Welsh, E., Fulp, W. et al. Differential association of STK11 and TP53 with KRAS mutation-associated gene expression, proliferation and immune surveillance in lung adenocarcinoma. Oncogene 35, 3209–3216 (2016). https://doi.org/10.1038/onc.2015.375
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.375
This article is cited by
-
NPAS2 dampens chemo-sensitivity of lung adenocarcinoma cells by enhancing DNA damage repair
Cell Death & Disease (2024)
-
Comprehensive analysis of co-expressed genes with TDP-43: prognostic and therapeutic potential in lung adenocarcinoma
Journal of Cancer Research and Clinical Oncology (2024)
-
Pancancer network analysis reveals key master regulators for cancer invasiveness
Journal of Translational Medicine (2023)
-
m6A methylation reader IGF2BP2 activates endothelial cells to promote angiogenesis and metastasis of lung adenocarcinoma
Molecular Cancer (2023)
-
Identification of four metabolic subtypes and key prognostic markers in lung adenocarcinoma based on glycolytic and glutaminolytic pathways
BMC Cancer (2023)