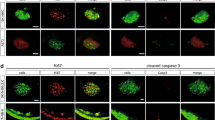
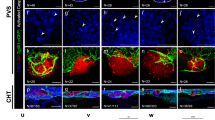
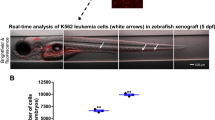
Abstract
High-throughput screens (HTS) of compound toxicity against cancer cells can identify thousands of potential new drug-leads. But only limited numbers of these compounds can progress to expensive and labor-intensive efficacy studies in mice, creating a ‘bottle neck’ in the drug development pipeline. Approaches that triage drug-leads for further study are greatly needed. Here we provide an intermediary platform between HTS and mice by adapting mouse models of pediatric brain tumors to grow as orthotopic xenografts in the brains of zebrafish. Freshly isolated mouse ependymoma, glioma and choroid plexus carcinoma cells expressing red fluorescence protein were conditioned to grow at 34 °C. Conditioned tumor cells were then transplanted orthotopically into the brains of zebrafish acclimatized to ambient temperatures of 34 °C. Live in vivo fluorescence imaging identified robust, quantifiable and reproducible brain tumor growth as well as spinal metastasis in zebrafish. All tumor xenografts in zebrafish retained the histological characteristics of the corresponding parent mouse tumor and efficiently recruited fish endothelial cells to form a tumor vasculature. Finally, by treating zebrafish harboring ERBB2-driven gliomas with an appropriate cytotoxic chemotherapy (5-fluorouracil) or tyrosine kinase inhibitor (erlotinib), we show that these models can effectively assess drug efficacy. Our data demonstrate, for the first time, that mouse brain tumors can grow orthotopically in fish and serve as a platform to study drug efficacy. As large cohorts of brain tumor-bearing zebrafish can be generated rapidly and inexpensively, these models may serve as a powerful tool to triage drug-leads from HTS for formal efficacy testing in mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O'Leary M et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 2010; 28: 2625–2634.
Kawauchi D, Robinson G, Uziel T, Gibson P, Rehg J, Gao C et al. A mouse model of the most aggressive subgroup of human medulloblastoma. Cancer Cell 2012; 21: 168–180.
Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C et al. Subtypes of medulloblastoma have distinct developmental origins. Nature 2010; 468: 1095–1099.
Pei Y, Moore Colin E, Wang J, Tewari Alok K, Eroshkin A, Cho Y-J et al. An Animal Model of MYC-Driven Medulloblastoma. Cancer Cell 2012; 21: 155–167.
Swartling FJ, Grimmer MR, Hackett CS, Northcott PA, Fan Q-W, Goldenberg DD et al. Pleiotropic role for MYCN in medulloblastoma. Genes Dev 2010; 24: 1059–1072.
Chow Lionel ML, Endersby R, Zhu X, Rankin S, Qu C, Zhang J et al. Cooperativity within and among Pten, p53, and Rb pathways induces high-grade astrocytoma in adult brain. Cancer Cell 2011; 19: 305–316.
Johnson RA, Wright KD, Poppleton H, Mohankumar KM, Finkelstein D, Pounds SB et al. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature 2010; 466: 632–636.
Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, Baines H et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/-)p53(-/-) mice. Cancer Cell 2004; 6: 229–240.
Atkinson Jennifer M, Shelat Anang A, Carcaboso Angel M, Kranenburg Tanya A, Arnold LA, Boulos N et al. An integrated in vitro and in vivo high-throughput screen identifies treatment leads for ependymoma. Cancer Cell 2011; 20: 384–399.
Caponigro G, Sellers WR . Advances in the preclinical testing of cancer therapeutic hypotheses. Nat Rev Drug Discov 2011; 10: 179–187.
Macarron R, Banks MN, Bojanic D, Burns DJ, Cirovic DA, Garyantes T et al. Impact of high-throughput screening in biomedical research. Nat Rev Drug Discov 2011; 10: 188–195.
White R, Rose K, Zon L . Zebrafish cancer: the state of the art and the path forward. Nat Rev Cancer 2013; 13: 624–636.
Stoletov K, Klemke R . Catch of the day: zebrafish as a human cancer model. Oncogene 2008; 27: 4509–4520.
Herbert J, Cavallaro T, Dwork AJ . A marker for primary choroid plexus neoplasms. Am J Pathol 1990; 136: 1317–1325.
Hanahan D, Weinberg Robert A . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Lawson ND, Weinstein BM . In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol 2002; 248: 307–318.
Stoletov K, Montel V, Lester RD, Gonias SL, Klemke R . High-resolution imaging of the dynamic tumor cell–vascular interface in transparent zebrafish. Proc Natl Acad Sci USA 2007; 104: 17406–17411.
Pounds S, Gao CL, Johnson RA, Wright KD, Poppleton H, Finkelstein D et al. A procedure to statistically evaluate agreement of differential expression for cross-species genomics. Bioinformatics 2011; 27: 2098–2103.
Walbert T, Gilbert M, Groves M, Puduvalli V, Alfred Yung WK, Conrad C et al. Combination of 6-thioguanine, capecitabine, and celecoxib with temozolomide or lomustine for recurrent high-grade glioma. J Neurooncol 2011; 102: 273–280.
Prados MD, Lamborn KR, Chang S, Burton E, Butowski N, Malec M et al. Phase 1 study of erlotinib HCl alone and combined with temozolomide in patients with stable or recurrent malignant glioma. Neuro Oncol 2006; 8: 67–78.
TCGA. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008; 455: 1061–1068.
Collymore C, Rasmussen S, Tolwani RJ . Gavaging adult zebrafish. J Vis Exp 2013; 11: e50691.
Hernan R, Fasheh R, Calabrese C, Frank AJ, Maclean KH, Allard D et al. ERBB2 up-regulates S100A4 and several other prometastatic genes in medulloblastoma. Cancer Res 2003; 63: 140–148.
Lacy J, Saadati H, Yu JB . Complications of brain tumors and their treatment. Hematol Oncol Clin North Am 2012; 26: 779–796.
Lin NU, Wefel JS, Lee EQ, Schiff D, van den Bent MJ, Soffietti R et al. Challenges relating to solid tumour brain metastases in clinical trials, part 2: neurocognitive, neurological, and quality-of-life outcomes. A report from the RANO group. Lancet Oncol 2013; 14: e407–e416.
White RM, Cech J, Ratanasirintrawoot S, Lin CY, Rahl PB, Burke CJ et al. DHODH modulates transcriptional elongation in the neural crest and melanoma. Nature 2011; 471: 518–522.
Hong S-K, Tsang M, Dawid IB . The mych gene is required for neural crest survival during zebrafish development. PLoS One 2008; 3: e2029.
Ridges S, Heaton WL, Joshi D, Choi H, Eiring A, Batchelor L et al. Zebrafish screen identifies novel compound with selective toxicity against leukemia. Blood 2012; 119: 5621–5631.
Wienholds E, Schulte-Merker S, Walderich B, Plasterk RH . Target-selected inactivation of the zebrafish rag1 gene. Science 2002; 297: 99–102.
White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2008; 2: 183–189.
Acknowledgements
This work was supported by grants from the National Institutes of Health (R01CA129541, P01CA96832 and P30CA021765, RJG), the Collaborative Ependymoma Research Network (CERN) and by ALSAC. We are grateful to the staff of the Hartwell Center for Bioinformatics and Biotechnology, and Flow Cytometry & Cell Sorting Shared Resource at St Jude Children’s Research Hospital for technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Eden, C., Ju, B., Murugesan, M. et al. Orthotopic models of pediatric brain tumors in zebrafish. Oncogene 34, 1736–1742 (2015). https://doi.org/10.1038/onc.2014.107
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.107
This article is cited by
-
Intravital imaging of metastasis in adult Zebrafish
BMC Cancer (2017)
-
Management and potentialities of primary cancer cultures in preclinical and translational studies
Journal of Translational Medicine (2017)
-
Fishing for cures: The alLURE of using zebrafish to develop precision oncology therapies
npj Precision Oncology (2017)
-
Stable multilineage xenogeneic replacement of definitive hematopoiesis in adult zebrafish
Scientific Reports (2016)