Abstract
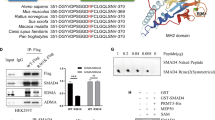
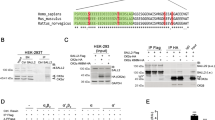
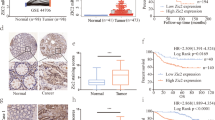
Dysregulation of cellular signaling pathways can lead to colon cancer. However, research on the key signaling effectors or regulators in colon carcinogenesis is limited. Casein kinase-2 interacting protein-1 (CKIP-1; also known as PLEKHO1) is crucial during adult bone formation and is a promising drug target for osteoporosis therapy. In this study, we observed that CKIP-1 was downregulated in human colon cancer tissues and colon cancer cell lines, and this result was correlated with colon cancer progression. CKIP-1 silencing in colon cancers involved promoter methylation. In colon cancer HCT116 and SW480 cells, CKIP-1 overexpression inhibited cell growth and migration. CKIP-1 also suppressed in-vivo tumor formation. Notably, the growth-suppressive role of CKIP-1 was dependent on the downregulation of the cell cycle-regulated oncogene Smad ubiquitylation regulatory factor-1 (Smurf1). During cell cycle progression, phosphatidylinositol-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling increased Smurf1 production by an mTOR-dependent translational control mechanism. Rapamycin, the mTOR inhibitor, significantly reduced Smurf1 protein levels, and Smurf1 was degraded in mitosis. In colon cancer, CKIP-1 controlled Smurf1 expression by suppressing PI3K/Akt/mTOR signaling and enhancing Smurf1 autodegradation, and CKIP-1 downregulation was correlated with Smurf1 upregulation in colon carcinogenesis. These findings provide novel insight into the mechanisms of the candidate tumor suppressor CKIP-1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Abbreviations
- BMP:
-
bone morphogenetic protein
- CKIP-1:
-
casein kinase-2 interacting protein-1
- EGFR:
-
epidermal growth factor receptor
- GSK3:
-
glycogen synthase kinase-3
- mTOR:
-
mammalian target of rapamycin
- PI3K:
-
phosphatidylinositol-3-kinase
- Smurf1:
-
Smad ubiquitylation regulatory factor-1
- TGF-β:
-
transforming growth factor-β.
References
Bosc DG, Graham KC, Saulnier RB, Zhang CJ, Prober D, Gietz RD et al. Identification and characterization of CKIP-1, a novel pleckstrin homology domain-containing protein that interacts with protein kinase CK2. J Biol Chem 2000; 275: 14295–14306.
Canton DA, Olsten MEK, Niederstrasser H, Cooper JA, Litchfield DW . The role of CKIP-1 in cell morphology depends on its interaction with actin-capping protein. J Biol Chem 2006; 281: 36347–36359.
Safi A, Vandromme M, Caussanel S, Valdacci L, Baas D, Vidal M et al. Role for the pleckstrin homology domain-containing protein CKIP-1 in phosphatidylinositol 3-kinase-regulated muscle differentiation. Mol Cell Biol 2004; 24: 1245–1255.
Zhang L, Tang Y, Tie Y, Tian C, Wang J, Dong Y et al. The PH domain containing protein CKIP-1 binds to IFP35 and Nmi and is involved in cytokine signaling. Cell Signal 2007; 19: 932–944.
Lu K, Yin X, Weng T, Xi S, Li L, Xing G et al. Targeting WW domains linker of HECT-type ubiquitin ligase Smurf1 for activation by CKIP-1. Nat Cell Biol 2008; 10: 994–1002.
Zhang L, Xing G, Tie Y, Tang Y, Tian C, Li L et al. Role for the pleckstrin homology domain-containing protein CKIP-1 in AP-1 regulation and apoptosis. EMBO J 2005; 24: 766–778.
Tokuda E, Fujita N, Oh-hara T, Sato S, Kurata A, Katayama R et al. Casein kinase 2-interacting protein-1, a novel Akt pleckstrin homology domain-interacting protein, down-regulates PI3K/Akt signaling and suppresses tumor growth in vivo. Cancer Res 2007; 67: 9666–9676.
Zhang G, Guo B, Wu H, Tang T, Zhang BT, Zheng L et al. A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nat Med 2012; 18: 307–314.
Jemal A, Siegel R, Xu J, Ward E . Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277–300.
Lammi L, Arte S, Somer M, Jarvinen H, Lahermo P, Thesleff I et al. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet 2004; 74: 1043–1050.
Li H, Pamukcu R, Thompson WJ . beta-Catenin signaling: therapeutic strategies in oncology. Cancer Biol Ther 2002; 1: 621–625.
Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 1997; 275: 1787–1790.
Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB . Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 2005; 4: 988–1004.
Zhang B, Halder S, Kashikar N, Cho Y, Datta A, Gorden DL et al. Antimetastatic role of Smad4 signaling in colorectal cancer. Gastroenterology 2010; 138: 969–980.
Wang Y, Nie J, Zhang L, Lu K, Xing G, Xie P et al. CKIP-1 couples Smurf1 ubiquitin ligase with Rpt6 subunit of proteasome to promote substrate degradation. EMBO Rep 2012; 13: 1004–1011.
Yamashita M, Ying SX, Zhang GM, Li CL, Cheng SY, Deng CX et al. Ubiquitin ligase Smurf1 controls osteoblast activity and bone homeostasis by targeting MEKK2 for degradation. Cell 2005; 121: 101–113.
Narimatsu M, Bose R, Pye M, Zhang L, Miller B, Ching P et al. Regulation of planar cell polarity by smurf ubiquitin ligases. Cell 2009; 137: 295–307.
Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL . Regulation of the polarity protein Par6 by TGF beta receptors controls epithelial cell plasticity. Science 2005; 307: 1603–1609.
Suzuki A, Shibata T, Shimada Y, Murakami Y, Horii A, Shiratori K et al. Identification of SMURF1 as a possible target for 7q21.3-22.1 amplification detected in a pancreatic cancer cell line by in-house array-based comparative genomic hybridization. Cancer Sci 2008; 99: 986–994.
Nie J, Liu L, Zhao X, Xie P, Zhou P, Xing G et al. DNA damage stress induces the dissociation of Smurf1/2 from MDM2 in a slow manner. Chinese Sci Bull 2011; 56: 3155–3161.
Cui Y, He S, Xing C, Lu K, Wang J, Xing G et al. SCFFBXL15 regulates BMP signalling by directing the degradation of HECT-type ubiquitin ligase Smurf1. EMBO J 2011; 30: 2675–2689.
Kannan M, Lee SJ, Schwedhelm-Domeyer N, Stegmuller J . The E3 ligase Cdh1-anaphase promoting complex operates upstream of the E3 ligase Smurf1 in the control of axon growth. Development 2012; 139: 3600–3612.
Nie J, Xie P, Liu L, Xing G, Chang Z, Yin Y et al. Smad ubiquitylation regulatory factor 1/2 (Smurf1/2) promotes p53 degradation by stabilizing the E3 ligase MDM2. J Biol Chem 2010; 285: 22818–22830.
Nie J, Liu L, Wu M, Xing G, He S, Yin Y et al. HECT ubiquitin ligase Smurf1 targets the tumor suppressor ING2 for ubiquitination and degradation. FEBS Lett 2010; 584: 3005–3012.
Osmundson EC, Ray D, Moore FE, Gao QS, Thomsen GH, Kiyokawa H . The HECT E3 ligase Smurf2 is required for Mad2-dependent spindle assembly checkpoint. J Cell Biol 2008; 183: 267–277.
Osmundson EC, Ray D, Moore FE, Kiyokawa H . Smurf2 as a novel mitotic regulator: From the spindle assembly checkpoint to tumorigenesis. Cell Div 2009; 4: 14.
Chen X, Zhang H, Wang H, Zhu J, Zhou WY, Zhang H . Transforming growth factor-β1 induces epithelial-to-mesenchymal transition in human lung cancer cells via PI3K/Akt and MEK/Erk1/2 signaling pathways. Mol Biol Rep 2012; 39: 3549–3556.
Acknowledgements
The excellent technical assistance of Qian Mei and Zhiqiang Wu is gratefully acknowledged. The study was supported by the National Basic Research Programs (2012CB910702, 2011CB910602 and 2012CB518103) and National Natural Science Foundation Projects (31125010, 31100554, 31270820, 31230061 and 81221004) and Beijing Nova Program (Z121107002512121).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Nie, J., Liu, L., Xing, G. et al. CKIP-1 acts as a colonic tumor suppressor by repressing oncogenic Smurf1 synthesis and promoting Smurf1 autodegradation. Oncogene 33, 3677–3687 (2014). https://doi.org/10.1038/onc.2013.340
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.340
Keywords
This article is cited by
-
SMURF1, a promoter of tumor cell progression?
Cancer Gene Therapy (2021)
-
Upregulation of CKIP-1 inhibits high-glucose induced inflammation and oxidative stress in HRECs and attenuates diabetic retinopathy by modulating Nrf2/ARE signaling pathway: an in vitro study
Cell & Bioscience (2019)
-
ERCC6L that is up-regulated in high grade of renal cell carcinoma enhances cell viability in vitro and promotes tumor growth in vivo potentially through modulating MAPK signalling pathway
Cancer Gene Therapy (2019)
-
Improvement of gemcitabine sensitivity of p53-mutated pancreatic cancer MiaPaCa-2 cells by RUNX2 depletion-mediated augmentation of TAp73-dependent cell death
Oncogenesis (2016)
-
Capping protein regulators fine-tune actin assembly dynamics
Nature Reviews Molecular Cell Biology (2014)