Abstract
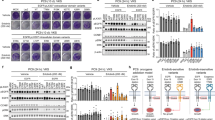
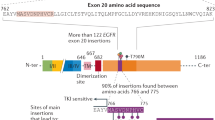
Activating mutations in the platelet-derived growth factor (PDGF) receptor alpha (PDGFRA) have been described in patients with gastrointestinal stromal tumors or myeloid malignancies associated with hypereosinophilia. These patients respond well to imatinib mesylate, raising the question as to whether patients with a PDGF receptor mutation in other tumor types should receive a tyrosine kinase inhibitor treatment. We characterized 10 novel somatic point mutations in PDGFRA that have been reported in isolated cases of glioblastoma, melanoma, acute myeloid leukemia, peripheral nerve sheath tumors and neuroendocrine carcinoma. The PDGFRA transmembrane domain mutation V536E stimulated Ba/F3 cell growth and signaling via ERK and STAT5 in the absence of ligand. This mutant, identified in glioblastoma, was strongly inhibited by imatinib. Modeling suggested that the mutation modulates the packing of the transmembrane domain helices in the receptor dimer. By contrast, two mutations in highly conserved residues affected the receptor traffic to the cell surface or kinase activity, thereby preventing the response to PDGF. The other mutations had no significant impact on the receptor activity. This functional analysis matched the predictions of SIFT and PolyPhen for only five mutations and these algorithms do not discriminate gain from loss of function. Finally, an E996K variant that had been identified in a melanoma cell line was not expressed in these cells. Altogether, several newly identified PDGFRA mutations do not activate the receptor and may therefore represent passenger mutations. Our results also underline the importance of characterizing novel kinase alterations in cancer patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Andrae J, Gallini R, Betsholtz C . Role of platelet-derived growth factors inphysiology and medicine. Genes Dev 2008; 22: 1276–1312.
Chiara F, Bishayee S, Heldin CH, Demoulin JB . Autoinhibition of the platelet-derived growth factor beta-receptor tyrosine kinase by its C-terminal tail. J Biol Chem 2004; 279: 19732–19738.
Toffalini F, Demoulin JB . New insights into the mechanisms of hematopoietic cell transformation by activated receptor tyrosine kinases. Blood 2010; 116: 2429–2437.
Corless CL, Schroeder A, Griffith D, Town A, McGreevey L, Harrell P et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol 2005; 23: 5357–5364.
Demoulin JB, Montano-Almendras CP . Platelet-derived growth factors and their receptors in normal and malignant hematopoiesis. Am J Blood Res 2012; 2: 44–56.
Medves S, Demoulin JB . Tyrosine kinase gene fusions in cancer: translating mechanisms into targeted therapies. J Cell Mol Med 2012; 16: 237–248.
Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med 2003; 348: 1201–1214.
Elling C, Erben P, Walz C, Frickenhaus M, Schemionek M, Stehling M et al. Novel imatinib-sensitive PDGFRA-activating point mutations in hypereosinophilicsyndrome induce growth factor independence and leukemia-like disease. Blood 2011; 117: 2935–2943.
Huss S, Wardelmann E, Goltz D, Binot E, Hartmann W, Merkelbach-Bruse S et al. Activating PDGFRA mutations in inflammatory fibroid polyps occur in exons 12, 14 and 18 and are associated with tumour localization. Histopathology 2012; 61: 59–68.
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastomacharacterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010; 17: 98–110.
Clarke ID, Dirks PB . A human brain tumor-derived PDGFR-alpha deletion mutant is transforming. Oncogene 2003; 22: 722–733.
Ozawa T, Brennan CW, Wang L, Squatrito M, Sasayama T, Nakada M et al. PDGFRA gene rearrangements are frequent genetic events in PDGFRA-amplifiedglioblastomas. Genes Dev 2010; 24: 2205–2218.
David M, Cross NC, Burgstaller S, Chase A, Curtis C, Dang R et al. Durable responses to imatinib in patients with PDGFRB fusion gene-positive and BCR-ABL-negative chronic myeloproliferative disorders. Blood 2007; 109: 61–64.
Metzgeroth G, Walz C, Erben P, Popp H, Schmitt-Graeff A, Haferlach C et al. Safety and efficacy of imatinib in chronic eosinophilic leukaemia and hypereosinophilic syndrome: a phase-II study. Br J Haematol 2008; 143: 707–715.
Paulsson J, Lindh MB, Jarvius M, Puputti M, Nister M, Nupponen NN et al. Prognostic but not predictive role of platelet-derived growth factor receptors in patients with recurrent glioblastoma. Int J Cancer 2011; 128: 1981–1988.
Dresemann G, Weller M, Rosenthal MA, Wedding U, Wagner W, Engel E et al. Imatinib in combination with hydroxyurea versus hydroxyurea alone as oral therapy in patients with progressive pretreated glioblastoma resistant to standard dose temozolomide. J Neurooncol 2010; 96: 393–402.
Holtkamp N, Okuducu AF, Mucha J, Afanasieva A, Hartmann C, Atallah I et al. Mutation and expression of PDGFRA and KIT in malignant peripheral nerve sheath tumors, and its implications for imatinib sensitivity. Carcinogenesis 2006; 27: 664–671.
Hiwatari M, Taki T, Tsuchida M, Hanada R, Hongo T, Sako M et al. Novel missense mutations in the tyrosine kinase domain of the platelet-derived growth factor receptor alpha(PDGFRA) gene in childhood acute myeloid leukemia with t(8;21)(q22;q22) or inv(16)(p13q22). Leukemia 2005; 19: 476–477.
Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G et al. Patterns of somatic mutation in human cancer genomes. Nature 2007; 446: 153–158.
Rand V, Huang J, Stockwell T, Ferriera S, Buzko O, Levy S et al. Sequence survey of receptor tyrosine kinases reveals mutations in glioblastomas. Proc Natl Acad Sci USA 2005; 102: 14344–14349.
Alentorn A, Marie Y, Carpentier C, Boisselier B, Giry M, Labussiere M et al. Prevalence, clinico-pathological value, and co-occurrence of PDGFRA abnormalities in diffuse gliomas. Neuro Oncol 2012; 14: 1393–1403.
Toffalini F, Hellberg C, Demoulin JB . Critical role of the platelet-derived growth factor receptor (PDGFR)-beta transmembrane domain in the TEL-PDGFRbeta cytosolic oncoprotein. J Biol Chem 2010; 285: 12268–12278.
Keating MT, Harryman CC, Williams LT . Platelet-derived growth factor receptor inducibility is acquired immediately after translation and does not requireglycosylation. J Biol Chem 1989; 264: 9129–9132.
Burke CL, Lemmon MA, Coren BA, Engelman DM, Stern DF . Dimerization of the p185neu transmembrane domain is necessary but not sufficient for transformation. Oncogene 1997; 14: 687–696.
Yang Y, Yuzawa S, Schlessinger J . Contacts between membrane proximal regions of the PDGF receptor ectodomain are required for receptor activation but not for receptor dimerization. Proc Natl Acad Sci USA 2008; 105: 7681–7686.
Polyansky AA, Volynsky PE, Efremov RG . Multistate organization of transmembrane helical protein dimers governed by the host membrane. J Am Chem Soc 2012; 134: 14390–14400.
Muhle-Goll C, Hoffmann S, Afonin S, Grage SL, Polyansky AA, Windisch D et al. Hydrophobic matching controls the tilt and stability of the dimeric platelet-derived growth factor receptor (PDGFR) beta transmembrane segment. J Biol Chem 2012; 287: 26178–26186.
Bell CA, Tynan JA, Hart KC, Meyer AN, Robertson SC, Donoghue DJ . Rotational coupling of the transmembrane and kinase domains of the Neu receptor tyrosine kinase. Mol Biol Cell 2000; 11: 3589–3599.
Arkhipov A, Shan Y, Das R, Endres NF, Eastwood MP, Wemmer DE et al. Architecture and membrane interactions of the EGF receptor. Cell 2013; 152: 557–569.
Endres NF, Das R, Smith AW, Arkhipov A, Kovacs E, Huang Y et al. Conformational coupling across the plasma membrane in activation of the EGF receptor. Cell 2013; 152: 543–556.
Yuzawa S, Opatowsky Y, Zhang Z, Mandiyan V, Lax I, Schlessinger J . Structural basis for activation of the receptor tyrosine kinase KIT by stem cell factor. Cell 2007; 130: 323–334.
Omura T, Heldin CH, Ostman A . Immunoglobulin-like domain 4-mediated receptor-receptor interactions contribute to platelet-derived growth factor-induced receptor dimerization. J Biol Chem 1997; 272: 12676–12682.
Schonherr C, Ruuth K, Eriksson T, Yamazaki Y, Ottmann C, Combaret V et al. The neuroblastoma ALK(I1250T) mutation is a kinase-dead RTK in vitro and in vivo. Transl Oncol 2011; 4: 258–265.
Demoulin JB, Seo JK, Ekman S, Grapengiesser E, Hellman U, Ronnstrand L et al. Ligand-induced recruitment of Na+/H+-exchanger regulatory factor to the PDGF (platelet-derived growth factor) receptor regulates actin cytoskeleton reorganization by PDGF. Biochem J 2003; 376: 505–510.
Takahashi Y, Morales FC, Kreimann EL, Georgescu MM . PTEN tumor suppressor associates with NHERF proteins to attenuate PDGF receptor signaling. EMBOJ 2006; 25: 910–920.
Frohling S, Scholl C, Levine RL, Loriaux M, Boggon TJ, Bernard OA et al. Identification of driver and passenger mutations of FLT3 by high-throughput DNA sequence analysis and functional assessment of candidate alleles. Cancer Cell 2007; 12: 501–513.
Ramensky V, Bork P, Sunyaev S . Human non-synonymous SNPs: server and survey. Nucleic Acids Res 2002; 30: 3894–3900.
Zhang J, Grubor V, Love CL, Banerjee A, Richards KL, Mieczkowski PA et al. Genetic heterogeneity of diffuse large B-cell lymphoma. Proc Natl Acad Sci USA 2013; 110: 1398–1403.
Toffalini F, Kallin A, Vandenberghe P, Pierre P, Michaux L, Cools J et al. The fusion proteins TEL-PDGFRbeta and FIP1L1-PDGFRalpha escape ubiquitination and degradation. Haematologica 2009; 94: 1085–1093.
Demoulin JB, Uyttenhove C, Van Roost E, de Lestre B, Donckers D, Van Snick J et al. A single tyrosine of the interleukin-9 (IL-9) receptor is required for STAT activation, antiapoptotic activity, and growth regulation by IL-9. Mol Cell Biol 1996; 16: 4710–4716.
Montano-Almendras CP, Essaghir A, Schoemans H, Varis I, Noel LA, Velghe AI et al. ETV6-PDGFRB and FIP1L1-PDGFRA stimulate human hematopoietic progenitor proliferation and differentiation into eosinophils: role of NF-kappaB. Haematologica 2012; 97: 1064–1072.
Demoulin JB, Ericsson J, Kallin A, Rorsman C, Ronnstrand L, Heldin CH . Platelet-derived growth factor stimulates membrane lipid synthesis through activation of phosphatidylinositol 3-kinase and sterol regulatory element-binding proteins. J Biol Chem 2004; 279: 35392–35402.
Schonherr C, Ruuth K, Kamaraj S, Wang CL, Yang HL, Combaret V et al. Anaplastic Lymphoma Kinase (ALK) regulates initiation of transcription of MYCN in neuroblastoma cells. Oncogene 2012; 31: 5193–5200.
Medves S, Noel LA, Montano-Almendras CP, Albu RI, Schoemans H, Constantinescu SN et al. Multiple oligomerization domains of KANK1-PDGFRB are required for JAK2-independent hematopoietic cell proliferation and signaling via STAT5 and ERK. Haematologica 2011; 96: 1406–1414.
Acknowledgements
We thank Francis Brasseur (Ludwig Institute for Cancer Research, Brussels, Belgium) for the LB373-MEL cell line and Stefan Constantinescu (de Duve Institute) for advices. This work was supported by the Salus Sanguinis Foundation and a grant from ‘Action de Recherches Concertées’ (Communauté Française de Belgique, Belgium).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Velghe, A., Van Cauwenberghe, S., Polyansky, A. et al. PDGFRA alterations in cancer: characterization of a gain-of-function V536E transmembrane mutant as well as loss-of-function and passenger mutations. Oncogene 33, 2568–2576 (2014). https://doi.org/10.1038/onc.2013.218
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.218
Keywords
This article is cited by
-
PDGFRA K385 mutants in myxoid glioneuronal tumors promote receptor dimerization and oncogenic signaling
Scientific Reports (2024)
-
High frequency of PDGFRA and MUC family gene mutations in diffuse hemispheric glioma, H3 G34-mutant: a glimmer of hope?
Journal of Translational Medicine (2022)
-
PDGF receptor mutations in human diseases
Cellular and Molecular Life Sciences (2021)
-
Genetic analyses in mouse fibroblast and melanoma cells demonstrate novel roles for PDGF-AB ligand and PDGF receptor alpha
Scientific Reports (2020)
-
Genome wide association and gene enrichment analysis reveal membrane anchoring and structural proteins associated with meat quality in beef
BMC Genomics (2019)