Abstract
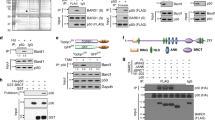
The BRCA1 tumor suppressor protein heterodimerizes with its partner protein, BARD1, via the RING domain present in both proteins. The heterodimer contains an E3 ubiquitin ligase activity and participates in multiple cellular functions such as cell cycle control, DNA repair and regulation of gene transcription, collectively aimed at maintaining genomic stability and tumor suppression. Yet, the precise role of BRCA1 E3 ligase in these cellular functions is poorly understood. We present data showing that BRCA1 ubiquitinates G2/M cell cycle proteins, cyclin B and Cdc25C, leading to their accelerated degradation via a mechanism that is independent of APC/C. BRCA1-dependent degradation of cyclin B and Cdc25C is reversed by proteasome inhibitors and is enhanced following DNA damage, which may represent a possible mechanism to prevent cyclin B and Cdc25C accumulation, a requirement for mitotic entry. Our data provide mechanistic insight into how BRCA1 E3 ligase activity regulates the G2/M cell cycle checkpoint and, thus, contributes to maintenance of genomic stability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Yarden RI, Papa MZ . BRCA1 at the crossroad of multiple cellular pathways: approaches for therapeutic interventions. Mol Cancer Ther 2006; 5: 1396–1404.
Lorick KL, Jensen JP, Fang S, Ong AM, Hatakeyama S, Weissman AM . RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc Natl Acad Sci USA 1999; 96: 11364–11369.
Mallery DL, Vandenberg CJ, Hiom K . Activation of the E3 ligase function of the BRCA1/BARD1 complex by polyubiquitin chains. Embo J 2002; 21: 6755–6762.
Xia Y, Pao GM, Chen HW, Verma IM, Hunter T . Enhancement of BRCA1 E3 ubiquitin ligase activity through direct interaction with the BARD1 protein. J Biol Chem 2003; 278: 5255–5263.
Yu X, Chini CC, He M, Mer G, Chen J . The BRCT domain is a phospho-protein binding domain. Science 2003; 302: 639–642.
Ruffner H, Joazeiro CA, Hemmati D, Hunter T, Verma IM . Cancer-predisposing mutations within the RING domain of BRCA1: loss of ubiquitin protein ligase activity and protection from radiation hypersensitivity. Proc Natl Acad Sci USA 2001; 98: 5134–5139.
Ohta T, Sato K, Wu W . The BRCA1 ubiquitin ligase and homologous recombination repair. FEBS Lett 2011; 585: 2836–2844.
Kim H, Huang J, Chen J . CCDC98 is a BRCA1-BRCT domain-binding protein involved in the DNA damage response. Nat Struct Mol Biol 2007; 14: 710–715.
Wang B, Matsuoka S, Ballif BA, Zhang D, Smogorzewska A, Gygi SP et al. Abraxas and RAP80 form a BRCA1 protein complex required for the DNA damage response. Science 2007; 316: 1194–1198.
Sobhian B, Shao G, Lilli DR, Culhane AC, Moreau LA, Xia B et al. RAP80 targets BRCA1 to specific ubiquitin structures at DNA damage sites. Science 2007; 316: 1198–1202.
Starita LM, Machida Y, Sankaran S, Elias JE, Griffin K, Schlegel BP et al. BRCA1-dependent ubiquitination of gamma-tubulin regulates centrosome number. Mol Cell Biol 2004; 24: 8457–8466.
Eakin CM, Maccoss MJ, Finney GL, Klevit RE . Estrogen receptor alpha is a putative substrate for the BRCA1 ubiquitin ligase. Proc Natl Acad Sci USA 2007; 104: 5794–5799.
Zhu Q, Pao GM, Huynh AM, Suh H, Tonnu N, Nederlof PM et al. BRCA1 tumour suppression occurs via heterochromatin-mediated silencing. Nature 2011; 477: 179–184.
Wu-Baer F, Lagrazon K, Yuan W, Baer R . The BRCA1/BARD1 heterodimer assembles polyubiquitin chains through an unconventional linkage involving lysine residue K6 of ubiquitin. J Biol Chem 2003; 278: 34743–34746.
Yu X, Fu S, Lai M, Baer R, Chen J . BRCA1 ubiquitinates its phosphorylation-dependent binding partner CtIP. Genes Dev 2006; 20: 1721–1726.
Sato K, Hayami R, Wu W, Nishikawa T, Nishikawa H, Okuda Y et al. Nucleophosmin/B23 is a candidate substrate for the BRCA1-BARD1 ubiquitin ligase. J Biol Chem 2004; 279: 30919–30922.
Wu W, Nishikawa H, Hayami R, Sato K, Honda A, Aratani S et al. BRCA1 ubiquitinates RPB8 in response to DNA damage. Cancer Res 2007; 67: 951–958.
Starita LM, Horwitz AA, Keogh MC, Ishioka C, Parvin JD, Chiba N . BRCA1/BARD1 ubiquitinate phosphorylated RNA polymerase II. J Biol Chem 2005; 280: 24498–24505.
Horwitz AA, Affar el B, Heine GF, Shi Y, Parvin JD . A mechanism for transcriptional repression dependent on the BRCA1 E3 ubiquitin ligase. Proc Natl Acad Sci USA 2007; 104: 6614–6619.
Poole AJ, Li Y, Kim Y, Lin SC, Lee WH, Lee EY . Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science 2006; 314: 1467–1470.
Calvo V, Beato M . BRCA1 counteracts progesterone action by ubiquitination leading to progesterone receptor degradation and epigenetic silencing of target promoters. Cancer Res 2011; 71: 3422–3431.
Yarden RI, Pardo-Reoyo S, Sgagias M, Cowan KH, Brody LC . BRCA1 regulates the G2/M checkpoint by activating Chk1 kinase upon DNA damage. Nat Genet 2002; 30: 285–289.
Yarden RI, Metsuyanim S, Pickholtz I, Shabbeer S, Tellio H, Papa MZ . BRCA1-dependent Chk1 phosphorylation triggers partial chromatin disassociation of phosphorylated Chk1 and facilitates S-phase cell cycle arrest. Int J Biochem Cell Biol 2012; 44: 1761–1769.
Hershko A . Mechanisms and regulation of the degradation of cyclin B. Philos Trans R Soc Lond B Biol Sci 1999; 354: 1571–1575 (discussion 5–6).
Acquaviva C, Pines J . The anaphase-promoting complex/cyclosome: APC/C. J Cell Sci 2006; 119 (Part 12): 2401–2404.
Chen F, Zhang Z, Bower J, Lu Y, Leonard SS, Ding M et al. Arsenite-induced Cdc25C degradation is through the KEN-box and ubiquitin-proteasome pathway. Proc Natl Acad Sci USA 2002; 99: 1990–1995.
Pfleger CM, Kirschner MW . The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev 2000; 14: 655–665.
Yan Y, Spieker RS, Kim M, Stoeger SM, Cowan KH . BRCA1-mediated G2/M cell cycle arrest requires ERK1/2 kinase activation. Oncogene 2005; 24: 3285–3296.
Aprelikova OPA, Fang B, Koller BH, Liu ET . BRCA1 is a selective co-activator of 14-3-3 sigma gene transcription in mouse embryonic stem cells. J Biol Chem 2001; 276: 25647–25650.
Au WW, Henderson BR . The BRCA1 RING and BRCT domains cooperate in targeting BRCA1 to ionizing radiation-induced nuclear foci. J Biol Chem 2005; 280: 6993–7001.
Yu X, Chen J . DNA damage-induced cell cycle checkpoint control requires CtIP, a phosphorylation-dependent binding partner of BRCA1 C-terminal domains. Mol Cell Biol 2004; 24: 9478–9486.
Xu B, O'Donnell AH, Kim ST, Kastan MB . Phosphorylation of serine 1387 in Brca1 is specifically required for the Atm-mediated S-phase checkpoint after ionizing irradiation. Cancer Res 2002; 62: 4588–4591.
Wu-Baer F, Ludwig T, Baer R . The UBXN1 protein associates with autoubiquitinated forms of the BRCA1 tumor suppressor and inhibits its enzymatic function. Mol Cell Biol 2011; 30: 2787–2798.
Christensen DE, Brzovic PS, Klevit RE . E2-BRCA1 RING interactions dictate synthesis of mono- or specific polyubiquitin chain linkages. Nat Struct Mol Biol 2007; 14: 941–948.
Brandeis M, Hunt T . The proteolysis of mitotic cyclins in mammalian cells persists from the end of mitosis until the onset of S phase. Embo J 1996; 15: 5280–5289.
Homer HA, McDougall A, Levasseur M, Murdoch AP, Herbert M . Mad2 is required for inhibiting securin and cyclin B degradation following spindle depolymerisation in meiosis I mouse oocytes. Reproduction 2005; 130: 829–843.
Wang RH, Yu H, Deng CX . A requirement for breast-cancer-associated gene 1 (BRCA1) in the spindle checkpoint. Proc Natl Acad Sci USA 2004; 101: 17108–17113.
Stegmeier F, Rape M, Draviam VM, Nalepa G, Sowa ME, Ang XL et al. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature 2007; 446: 876–881.
Innocente SA, Abrahamson JL, Cogswell JP, Lee JM . p53 regulates a G2 checkpoint through cyclin B1. Proc Natl Acad Sci USA 1999; 96: 2147–2152.
Jin P, Hardy S, Morgan DO . Nuclear localization of cyclin B1 controls mitotic entry after DNA damage. J Cell Biol 1998; 141: 875–885.
Toyoshima F, Moriguchi T, Wada A, Fukuda M, Nishida E . Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint. Embo J 1998; 17: 2728–2735.
Sato K, Sundaramoorthy E, Rajendra E, Hattori H, Jeyasekharan AD, Ayoub N et al. A DNA-damage selective role for BRCA1 E3 ligase in claspin ubiquitylation, CHK1 activation, and DNA repair. Curr Biol 2012; 22: 1659–1666.
Palacios J, Honrado E, Osorio A, Cazorla A, Sarrio D, Barroso A et al. Phenotypic characterization of BRCA1 and BRCA2 tumors based in a tissue microarray study with 37 immunohistochemical markers. Breast Cancer Res Treat 2005; 90: 5–14.
Morris JR, Boutell C, Keppler M, Densham R, Weekes D, Alamshah A et al. The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature 2009; 462: 886–890.
Reid LJ, Shakya R, Modi AP, Lokshin M, Cheng JT, Jasin M et al. E3 ligase activity of BRCA1 is not essential for mammalian cell viability or homology-directed repair of double-strand DNA breaks. Proc Natl Acad Sci USA 2008; 105: 20876–20881.
Shakya R, Reid LJ, Reczek CR, Cole F, Egli D, Lin CS et al. BRCA1 tumor suppression depends on BRCT phosphoprotein binding, but not its E3 ligase activity. Science 2011; 334: 525–528.
Brodie SG, Deng CX . BRCA1-associated tumorigenesis: what have we learned from knockout mice? Trends Genet 2001; 17: S18–S22.
Xu X, Qiao W, Linke SP, Cao L, Li WM, Furth PA et al. Genetic interactions between tumor suppressors Brca1 and p53 in apoptosis, cell cycle and tumorigenesis. Nat Genet 2001; 28: 266–271.
Wu W, Koike A, Takeshita T, Ohta T . The ubiquitin E3 ligase activity of BRCA1 and its biological functions. Cell Div 2008; 3: 1.
Ma Y, Fan S, Hu C, Meng Q, Fuqua SA, Pestell RG et al. BRCA1 regulates acetylation and ubiquitination of estrogen receptor-alpha. Mol Endocrinol 2010; 24: 76–90.
Lim KL, Chew KC, Tan JM, Wang C, Chung KK, Zhang Y et al. Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J Neurosci 2005; 25: 2002–2009.
Kirkpatrick DS, Hathaway NA, Hanna J, Elsasser S, Rush J, Finley D et al. Quantitative analysis of in vitro ubiquitinated cyclin B1 reveals complex chain topology. Nat Cell Biol 2006; 8: 700–710.
Yarden RI, Brody LC . BRCA1 interacts with components of the histone deacetylase complex. Proc Natl Acad Sci USA 1999; 96: 4983–4988.
Acknowledgements
We thank Drs B Henderson, J Pines, R Agami, E Rosen and M Brandeis for providing plasmids and Dr L Brody for reagents. We thank the microscopy unit in Bar-Ilan University for assistance with immunofluorescence studies and Dr I Goldstein and the flow cytometry unit at the Sheba medical center for assistance with fluorescence-activated cell sorting analysis. We thank Allison Hall for technical assistance. We thank Drs Eliot Rosen, Claire Pollack, Michael Brandeis and Gad Lavie for stimulating discussions and critical reading of the manuscript. This study was supported by a Research Career Development Award from the Israel Cancer Research Fund (to RIY) and by the funding from the Health Ministry (to RIY and MZP), the Israeli Cancer Association (RIY) and funds from Georgetown University, SNHS to RIY.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Shabbeer, S., Omer, D., Berneman, D. et al. BRCA1 targets G2/M cell cycle proteins for ubiquitination and proteasomal degradation. Oncogene 32, 5005–5016 (2013). https://doi.org/10.1038/onc.2012.522
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.522
Keywords
This article is cited by
-
Expression of tripartite motif-containing 44 and its prognostic and clinicopathological value in human malignancies:a meta-analysis
BMC Cancer (2020)
-
MiR-210-3p protects endometriotic cells from oxidative stress-induced cell cycle arrest by targeting BARD1
Cell Death & Disease (2019)
-
PALB2 connects BRCA1 and BRCA2 in the G2/M checkpoint response
Oncogene (2019)
-
Regulating BRCA1 protein stability by cathepsin S-mediated ubiquitin degradation
Cell Death & Differentiation (2019)
-
Cyclin B1 stability is increased by interaction with BRCA1, and its overexpression suppresses the progression of BRCA1-associated mammary tumors
Experimental & Molecular Medicine (2018)