Abstract
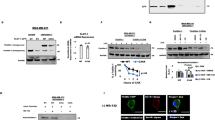
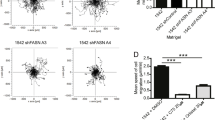
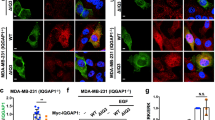
Protein tyrosine kinase 6 (PTK6) is a non-receptor tyrosine kinase expressed in epithelial cancers. Disruption of Ptk6 decreases azoxymethane-induced colon tumorigenesis in mice by preventing signal transducer and activator of transcription 3 activation. Relocalization of PTK6 in prostate cancers contributes to increased growth. Although not expressed in normal breast or ovary, PTK6 promotes anchorage-independent survival of breast and ovarian tumor cells. We identified several potential PTK6 substrates in the human SW620 colon cancer cell line using mass spectrometry, including FAK (focal adhesion kinase). We show that FAK is a direct substrate of PTK6 in vitro and in vivo. Expression of membrane-targeted active PTK6 (Palm-PTK6-YF) induces constitutive activation of FAK and cell morphology changes, which are independent of SRC family kinases in S rc−/−, Y es−/−, F yn−/− (SYF) mouse embryonic fibroblasts (MEFs). Palm-PTK6-YF expressing SYF cells are transformed and overcome contact inhibition, form colonies in transformation assays, proliferate in suspension and form tumors in a xenograft model. Expression of FAK and Palm-PTK6-YF in Fak−/− MEFs synergistically activates AKT and protects cells against anoikis. However, expression of Palm-PTK6-YF in Akt1/2−/− MEFs fails to protect cells from anoikis, indicating AKT is critical in PTK6 and FAK-mediated survival signaling. In a conditional Pten knockout murine prostate cancer model, we identify prostate epithelial cells with enhanced activation of endogenous PTK6 and FAK at the plasma membrane. Knockdown of PTK6 in the PC3 human prostate cancer cell line disrupts FAK and AKT activation and promotes anoikis, which can be rescued by exogenous expression of FAK. Our data reveal important roles for a PTK6-FAK-AKT signaling axis in promoting anchorage-independent cell survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mitchell PJ, Barker KT, Martindale JE, Kamalati T, Lowe PN, Page MJ et al. Cloning and characterisation of cDNAs encoding a novel non-receptor tyrosine kinase, brk, expressed in human breast tumours. Oncogene 1994; 9: 2383–2390.
Siyanova EY, Serfas MS, Mazo IA, Tyner AL . Tyrosine kinase gene expression in the mouse small intestine. Oncogene 1994; 9: 2053–2057.
Derry JJ, Richard S, Valderrama Carvajal H, Ye X, Vasioukhin V, Cochrane AW et al. Sik (BRK) phosphorylates Sam68 in the nucleus and negatively regulates its RNA binding ability. Mol Cell Biol 2000; 20: 6114–6126.
Derry JJ, Prins GS, Ray V, Tyner AL . Altered localization and activity of the intracellular tyrosine kinase BRK/Sik in prostate tumor cells. Oncogene 2003; 22: 4212–4220.
Zheng Y, Asara JM, Tyner AL . Protein-tyrosine kinase 6 promotes peripheral adhesion complex formation and cell migration by phosphorylating p130 CRK-associated substrate. J Biol Chem 2012; 287: 148–158.
Haegebarth A, Heap D, Bie W, Derry JJ, Richard S, Tyner AL . The nuclear tyrosine kinase BRK/Sik phosphorylates and inhibits the RNA-binding activities of the Sam68-like mammalian proteins SLM-1 and SLM-2. J Biol Chem 2004; 279: 54398–54404.
Liu L, Gao Y, Qiu H, Miller WT, Poli V, Reich NC . Identification of STAT3 as a specific substrate of breast tumor kinase. Oncogene 2006; 25: 4904–4912.
Weaver AM, Silva CM . Signal transducer and activator of transcription 5b: a new target of breast tumor kinase/protein tyrosine kinase 6. Breast Cancer Res 2007; 9: R79.
Palka-Hamblin HL, Gierut JJ, Bie W, Brauer PM, Zheng Y, Asara JM et al. Identification of {beta}-catenin as a target of the intracellular tyrosine kinase PTK6. J Cell Sci 2010; 123: 236–245.
Mitchell PJ, Sara EA, Crompton MR . A novel adaptor-like protein which is a substrate for the non-receptor tyrosine kinase, BRK. Oncogene 2000; 19: 4273–4282.
Chen HY, Shen CH, Tsai YT, Lin FC, Huang YP, Chen RH . Brk activates rac1 and promotes cell migration and invasion by phosphorylating paxillin. Mol Cell Biol 2004; 24: 10558–10572.
Li X, Lu Y, Liang K, Hsu J-M, Albarracin C, Mills GB et al. Brk/PTK6 sustains activated EGFR signaling through inhibiting EGFR degradation and transactivating EGFR. Oncogene e-pub ahead of print 9 January 2012 doi:10.1038/onc.2011.608.
Zheng Y, Peng M, Wang Z, Asara JM, Tyner AL . Protein tyrosine kinase 6 directly phosphorylates AKT and promotes AKT activation in response to epidermal growth factor. Mol Cell Biol 2010; 30: 4280–4292.
Shen CH, Chen HY, Lin MS, Li FY, Chang CC, Kuo ML et al. Breast tumor kinase phosphorylates p190RhoGAP to regulate rho and ras and promote breast carcinoma growth, migration, and invasion. Cancer Res 2008; 68: 7779–7787.
Barker KT, Jackson LE, Crompton MR . BRK tyrosine kinase expression in a high proportion of human breast carcinomas. Oncogene 1997; 15: 799–805.
Lofgren KA, Ostrander JH, Housa D, Hubbard GK, Locatelli A, Bliss RL et al. Mammary gland specific expression of Brk/PTK6 promotes delayed involution and tumor formation associated with activation of p38 MAPK. Breast Cancer Res 2011; 13: R89.
Llor X, Serfas MS, Bie W, Vasioukhin V, Polonskaia M, Derry J et al. BRK/Sik expression in the gastrointestinal tract and in colon tumors. Clin Cancer Res 1999; 5: 1767–1777.
Lin HS, Berry GJ, Fee WE, Terris DJ, Sun Z . Identification of tyrosine kinases overexpressed in head and neck cancer. Arch Otolaryngol Head Neck Surg 2004; 130: 311–316.
Schmandt RE, Bennett M, Clifford S, Thornton A, Jiang F, Broaddus RR et al. The BRK tyrosine kinase is expressed in high-grade serous carcinoma of the ovary. Cancer Biol Ther 2006; 5: 1136–1141.
Gierut J, Zheng Y, Bie W, Carroll RE, Ball-Kell S, Haegebarth A et al. Disruption of the mouse protein tyrosine kinase 6 gene prevents STAT3 activation and confers resistance to azoxymethane. Gastroenterology 2011; 141: 1371–1380, 80 e1-2.
Irie HY, Shrestha Y, Selfors LM, Frye F, Iida N, Wang Z et al. PTK6 regulates IGF-1-induced anchorage-independent survival. PLoS One 2010; 5: e11729.
Parsons JT . Focal adhesion kinase: the first ten years. J Cell Sci 2003; 116: 1409–1416.
Schaller MD . Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta 2001; 1540: 1–21.
Schlaepfer DD, Mitra SK . Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev 2004; 14: 92–101.
Xia H, Nho RS, Kahm J, Kleidon J, Henke CA . Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J Biol Chem 2004; 279: 33024–33034.
Zouq NK, Keeble JA, Lindsay J, Valentijn AJ, Zhang L, Mills D et al. FAK engages multiple pathways to maintain survival of fibroblasts and epithelia: differential roles for paxillin and p130Cas. J Cell Sci 2009; 122: 357–367.
Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K et al. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell 2008; 29: 9–22.
Manning BD, Cantley LC . AKT/PKB signaling: navigating downstream. Cell 2007; 129: 1261–1274.
Zhang X, Tang N, Hadden TJ, Rishi AK . Akt FoxO and regulation of apoptosis. Biochim Biophys Acta 2011; 1813: 1978–1986.
Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med 2002; 8: 1153–1160.
Rossig L, Jadidi AS, Urbich C, Badorff C, Zeiher AM, Dimmeler S . Akt-dependent phosphorylation of p21(Cip1) regulates PCNA binding and proliferation of endothelial cells. Mol Cell Biol 2001; 21: 5644–5657.
Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J . Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J 1997; 16: 2783–2793.
Guadamillas MC, Cerezo A, Del Pozo MA . Overcoming anoikis—pathways to anchorage-independent growth in cancer. J Cell Sci 2011; 124: 3189–3197.
Qiu H, Miller WT . Regulation of the nonreceptor tyrosine kinase Brk by autophosphorylation and by autoinhibition. J Biol Chem 2002; 277: 34634–34641.
Lukong KE, Huot ME, Richard S . BRK phosphorylates PSF promoting its cytoplasmic localization and cell cycle arrest. Cell Signal 2009; 21: 1415–1422.
Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY . Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol 1996; 134: 793–799.
Xu LH, Yang X, Bradham CA, Brenner DA, Baldwin AS, Craven RJ et al. The focal adhesion kinase suppresses transformation-associated, anchorage-independent apoptosis in human breast cancer cells. Involvement of death receptor-related signaling pathways. J Biol Chem 2000; 275: 30597–30604.
Calalb MB, Polte TR, Hanks SK . Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol 1995; 15: 954–963.
Song G, Ouyang G, Bao S . The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med 2005; 9: 59–71.
Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A et al. Pten dose dictates cancer progression in the prostate. PLoS Biol 2003; 1: E59.
Bouchard V, Demers MJ, Thibodeau S, Laquerre V, Fujita N, Tsuruo T et al. Fak/Src signaling in human intestinal epithelial cell survival and anoikis: differentiation state-specific uncoupling with the PI3-K/Akt-1 and MEK/Erk pathways. J Cell Physiol 2007; 212: 717–728.
Serfas MS, Tyner AL . Brk, Srm, Frk, and Src42A form a distinct family of intracellular Src-like tyrosine kinases. Oncol Res 2003; 13: 409–419.
Xiang B, Chatti K, Qiu H, Lakshmi B, Krasnitz A, Hicks J et al. Brk is coamplified with ErbB2 to promote proliferation in breast cancer. Proc Natl Acad Sci USA 2008; 105: 12463–12468.
Castro NE, Lange CA . Breast tumor kinase and extracellular signal-regulated kinase 5 mediate Met receptor signaling to cell migration in breast cancer cells. Breast Cancer Res 2010; 12: R60.
Brauer PM, Zheng Y, Wang L, Tyner AL . Cytoplasmic retention of protein tyrosine kinase 6 promotes growth of prostate tumor cells. Cell Cycle 2010; 9: 4190–4199.
Sakamoto S, Kyprianou N . Targeting anoikis resistance in prostate cancer metastasis. Mol Aspects Med 2010; 31: 205–214.
Desgrosellier JS, Cheresh DA . Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 2010; 10: 9–22.
Wu FY, Wang SE, Sanders ME, Shin I, Rojo F, Baselga J et al. Reduction of cytosolic p27(Kip1) inhibits cancer cell motility, survival, and tumorigenicity. Cancer Res 2006; 66: 2162–2172.
Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I et al. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell 2008; 14: 458–470.
Rasband WS . Image J. U S National Institutes of Health, Bethesda, Maryland, USA, 1997-2011. http://imagej.nih.gov/ij/.
Acknowledgements
This work was supported by NIH Grants 5R01DK044525 (ALT), 5P01CA120964 (JMA) and NIH DF/HCC Cancer Center Support Grant 5P30CA006516 (JMA). We thank Wenjun Bie and Min Yuan for technical assistance, Dr Nissim Hay (University of Illinois at Chicago, Chicago, IL, USA) for his gifts of Akt1/2−/− MEFs and PTENfl/fl, Probasin-Cre and control mouse tissues, and Dr David D Schlaepfer (University of California, San Diego, CA, USA) for providing FAK expression constructs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Zheng, Y., Gierut, J., Wang, Z. et al. Protein tyrosine kinase 6 protects cells from anoikis by directly phosphorylating focal adhesion kinase and activating AKT. Oncogene 32, 4304–4312 (2013). https://doi.org/10.1038/onc.2012.427
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.427
Keywords
This article is cited by
-
PTEN is a protein phosphatase that targets active PTK6 and inhibits PTK6 oncogenic signaling in prostate cancer
Nature Communications (2017)
-
Protein Tyrosine Kinase 6 Regulates UVB-Induced Signaling and Tumorigenesis in Mouse Skin
Journal of Investigative Dermatology (2015)
-
Protein tyrosine kinase 6 promotes ERBB2-induced mammary gland tumorigenesis in the mouse
Cell Death & Disease (2015)
-
The virus-induced protein APOBEC3G inhibits anoikis by activation of Akt kinase in pancreatic cancer cells
Scientific Reports (2015)
-
Cancer cell survival during detachment from the ECM: multiple barriers to tumour progression
Nature Reviews Cancer (2014)