Abstract
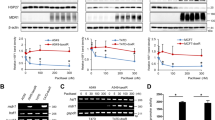
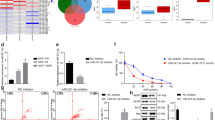
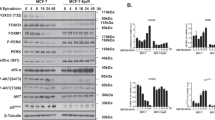
Resistance to chemotherapeutic drugs is a significant clinical problem in the treatment of cancer and this resistance has been linked to the cellular expression of multidrug-efflux transporters. The aim of this study was to explore the role of HOXC6 in the regulation of multidrug resistance (MDR) to chemotherapeutic drugs. The HOXC6 gene was identified as being overexpressed in drug-resistant cells compared with parental cell lines. Transfection assays demonstrated that HOXC6 activated MDR-1 promoter activity. A series of MDR-1 promoter deletion mutants was examined and the minimal HOXC6-responsive region was identified to be in the TAAT motif (−2243 bp) of the MDR-1 promoter. Interestingly, overexpression of HOXC6 in the parental cell lines resulted in the upregulation of MDR-1 expression. The inhibition of HOXC6 using small interfering RNA led to the repression of MDR-1. We determined that knockdown of HOXC6 expression in MDR cells increased their sensitivity to paclitaxel. Flow cytometry analysis suggested that siHOXC6 could induce paclitaxel-induced apoptosis and that this was accompanied by an increased accumulation and a decreased release of paclitaxel. Taken together, our findings suggest that HOXC6 expression is an important mechanism of chemotherapeutic drug resistance via its regulation of MDR-1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Abate-Shen C . Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer 2002; 2: 777–785.
Veraksa A, Del Campo M, McGinnis W . Developmental patterning genes and their conserved functions: from model organisms to humans. Mol Genet Metab 2000; 69: 85–100.
Friedmann Y, Daniel CA, Strickland P, Daniel CW . Hox genes in normal and neoplastic mouse mammary gland. Cancer Res 1994; 54: 5981–5985.
Castronovo V, Kusaka M, Chariot A, Gielen J, Sobel M . Homeobox genes: potential candidates for the transcriptional control of the transformed and invasive phenotype. Biochem Pharmacol 1994; 47: 137–143.
Chen H, Sukumar S . HOX genes: emerging stars in cancer. Cancer Biol Ther 2003; 2: 524–525.
Bodey B, Bodey B, Siegel SE, Kaiser HE . Immunocytochemical detection of the homeobox B3, B4, and C6 gene products in breast carcinomas. Anticancer Res 2000; 20: 3281–3286.
Castronovo V, Kusaka M, Chariot A, Gielen J, Sobel M . Homeobox genes: potential candidates for the transcriptional control of the transformed and invasive phenotype. Biochem Pharmacol 1994; 47: 137–143.
Fujiki K, Duerr EM, Kikuchi H, Ng A, Xavier RJ, Mizukami Y et al. Hoxc6 is overexpressed in gastrointestinal carcinoids and interacts with JunD to regulate tumor growth. Gastroenterology 2008; 135: 907–916.
Bodey B, Bodey BJR, Siegel SE, Luck JV, Kaiser HE . Homeobox B3, B4, and C6 gene product expression in osteosarcomas as detected by immunocytochemistry. Anticancer Res 2000; 20: 2717–2721.
Miller GJ, Miller HL, van Bokhoven A, Lambert JR, Werahera PN, Schirripa O et al. Aberrant HOXC expression accompanies the malignant phenotype in human prostate. Cancer Res 2003; 63: 5879–5888.
Garcia-Gasca A, Spyropoulos DD . Differential mammary morphogenesis along the anteroposterior axis in Hoxc6 gene targeted mice. Dev Dyn 2000; 219: 261–276.
Ramachandran S, Liu P, Young AN, Yin-Goen Q, Lim SD, Laycock N et al. Loss of HOXC6 expression induces apoptosis in prostate cancer cells. Oncogene 2005; 24: 188–198.
McCabe CD, Spyropoulos DD, Martin D, Moreno CS . Genome-wide analysis of the homeobox C6 transcriptional network in prostate cancer. Cancer Res 2008; 68: 1988–1996.
Grishina IB, Kim SY, Ferrara C, Makarenkova HP, Walden PD . BMP7 inhibits branching morphogenesis in the prostate gland and interferes with Notch signaling. Dev Biol 2005; 288: 334–347.
Ricort JM, Binoux M . Insulin-like growth factor-binding protein-3 activates a phosphotyrosine phosphatase. Effects on the insulin-like growth factor signaling pathway. J Biol Chem 2002; 277: 19448–19454.
Van der Geer P, Hunter T, Lindberg RA . Receptor protein-tyrosine kinases and their signal transduction pathways. Annu Rev Cell Biol 1994; 10: 251–337.
Lin Y, Liu G, Zhang Y, Hu YP, Yu K, Lin C et al. Fibroblast growth factor receptor 2 tyrosine kinase is required for prostatic morphogenesis and the acquisition of strict androgen dependency for adult tissue homeostasis. Development 2007; 134: 723–734.
Gottesman MM, Fojo T, Bates SE . Multidrug resistance in cancer: role of ATP dependent transporters. Nat Rev Cancer 2002; 2: 48–58.
Haimeur A, Conseil G, Deeley RG, Cole SP . The MRP-related and BCRP/ABCG2 multidrug resistance proteins: Biology, substrate specificity and regulation. Curr Drug Metab 2004; 5: 21–53.
Szakacs G, Annereau JP, Lababidi S, Shankavaram U, Arciello A, Bussey KJ et al. Predicting drug sensitivity and resistance: profiling ABC transporter genes in cancer cells. Cancer Cell 2004; 6: 129–137.
Deeley RG, Cole SP . Substrate recognition and transport by multidrug resistance protein 1 (ABCC1). FEBS Lett 2006; 580: 1103–1111.
Litman T, Druley TE, Stein WD, Bates SE . From MDR to MXR: new understanding of multidrug resistance systems, their properties and clinical significance. Cell Mol Life Sci 2001; 58: 931–959.
Cho S, Lu M, He X, Ee PL, Bhat U, Schneider E et al. Notch1 regulates the expression of the multidrug resistance gene ABCC1/MRP1 in cultured cancer cells. Proc Natl Acad Sci USA 2011; 108: 20778–20783.
Ernst T, Hergenhahn M, Kenzelmann M, Cohen CD, Bonrouhi M, Weninger A et al. Decrease and gain of gene expression are equally discriminatory markers for prostate carcinoma: a gene expression analysis on total and microdissected prostate tissue. Am J Pathol 2002; 160: 2169–2180.
De Vita G, Barba P, Odartchenko N, Givel JC, Freschi G, Bucciarelli G et al. Expression of homeobox-containing genes in primary and metastatic colorectal cancer. Eur J Cancer 1993; 29: 887–893.
Tiberio C, Barba P, Magli MC, Arvelo F, Le Chevalier T, Poupon MF et al. HOX gene expression in human small-cell lung cancers xenografted into nude mice. Int J Cancer 1994; 58: 608–615.
Svingen T, Tonissen KF . Hox transcription factors and their elusive mammalian gene targets. Heredity 2006; 97: 88–96.
Jones FS, Holst BD, Minowa O, De Robertis EM, Edelman GM . Binding and transcriptional activation of the promoter for the neural cell adhesion molecule by HoxC6 (Hox-3.3). Proc Natl Acad Sci USA 1993; 90: 6557–6561.
Kim SA, Yoon JH, Ahn SG . Heat shock factor 4a (HSF4a) represses HSF2 expression and HSF2-mediated transcriptional activity. J Cell Physiol 2012; 227: 1–6.
Guo C, Ding J, Yao L, Sun L, Lin T, Song Y et al. Tumor suppressor gene Runx3 sensitizes gastric cancer cells to chemotherapeutic drugs by downregulating Bcl-2, MDR-1 and MRP-1. Int J Cancer 2005; 116: 155–160.
Acknowledgements
This research was supported by National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. R13-2008-010-00000-0; No. 2011-0029091).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Kim, KJ., Moon, SM., Kim, SA. et al. Transcriptional regulation of MDR-1 by HOXC6 in multidrug-resistant cells. Oncogene 32, 3339–3349 (2013). https://doi.org/10.1038/onc.2012.354
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.354
Keywords
This article is cited by
-
HomeoboxC6 promotes metastasis by orchestrating the DKK1/Wnt/β-catenin axis in right-sided colon cancer
Cell Death & Disease (2021)
-
A forward genetic screen identifies modifiers of rocaglate responsiveness
Scientific Reports (2021)