Abstract
Genetic alterations of 16q21-q22, the locus of a 6-cadherin cluster, are frequently involved in multiple tumors, suggesting the presence of critical tumor suppressor genes (TSGs). Using 1 Mb array comparative genomic hybridization (aCGH), we refined a small hemizygous deletion (∼1 Mb) at 16q21-22.1, which contains a single gene Cadherin-11 (CDH11, OB-cadherin). CDH11 was broadly expressed in human normal adult and fetal tissues, while its silencing and promoter CpG methylation were frequently detected in tumor cell lines, but not in immortalized normal epithelial cells. Aberrant methylation was also frequently detected in multiple primary tumors. CDH11 silencing could be reversed by pharmacologic or genetic demethylation, indicating an epigenetic mechanism. Ectopic expression of CDH11 strongly suppressed tumorigenecity and induced tumor cell apoptosis. Moreover, CDH11 was found to inhibit Wnt/β-catenin and AKT/Rho A signaling, as well as actin stress fiber formation, thus further inhibiting tumor cell migration and invasion. CDH11 also inhibited epithelial-to-mesenchymal transition and downregulated stem cell markers. Thus, our work identifies CDH11 as a functional tumor suppressor and an important antagonist of Wnt/β-catenin and AKT/Rho A signaling, with frequent epigenetic inactivation in common carcinomas.
Similar content being viewed by others
Introduction
Various genetic analyses using microsatellite markers have demonstrated the frequent loss of heterozygosity at 16q in multiple tumors, including nasopharyngeal carcinoma (NPC; ∼40%) (Hui et al., 1999), hepatocellular carcinoma (HCC; ∼50%) (Yeh et al., 1996), breast cancer (∼77%) (Callen et al., 2002), lung caner (∼60%) (Stanton et al., 2000), prostate cancer (∼53%) (Suzuki et al., 1996) and gastric cancer (∼45%) (Nishioka et al., 2001). One critical deletion region has been mapped to 16q22.1-16q24.3, suggesting the presence of candidate tumor suppressor gene(s) (TSG). Several candidate TSGs have already been identified in this region, including WWOX (Aqeilan et al., 2007), CBFA2T3 (Kochetkova et al., 2002), ATBF1 (Sun et al., 2005), CMTM3 (Wang et al., 2009) and E-cadherin (CDH1) (Margulis et al., 2005).
Cadherins comprise an important group of cell–cell adhesion molecules that mediate intercellular adhesion by Ca2+-dependent homophilic interactions (Yagi and Takeichi, 2000). By forming homodimers, cadherins can cluster through a zipper-like mechanism, while their intracellular domain is anchored to the actin cytoskeleton through α-catenin and β-catenin (Angst et al., 2001). These interactions have crucial roles in maintaining tissue architecture and cell polarity, as well as limiting cell movement and proliferation, thus resulting in tumor inhibition (Berx and van Roy, 2009). Six classical cadherin family members, including CDH1, CDH3 (P-cadherin), CDH5 (VE-Cadherin), CDH8, CDH11 (OB-cadherin) and CDH13 (H-cadherin), are located on 16q22.1-16q24.3, as a so-called six-cadherin cluster (Kremmidiotis et al., 1998). Some cadherins have been identified as functional tumor suppressors, such as CDH1 (Margulis et al., 2005) and CDH13/H-cadherin (Andreeva and Kutuzov, 2010), involved in inhibiting cell proliferation and invasiveness, and promoting apoptosis.
Epigenetic alterations of TSGs, including promoter CpG methylation and histone modifications, are frequently involved in tumor development and progression (Bird, 2002). Remarkably, epigenetic silencing of CDH1 (Hiraguri et al., 1998; Eads et al., 2001; Wheeler et al., 2001; Kroeger et al., 2008) and CDH13 (Toyooka et al., 2001, 2002; Roman-Gomez et al., 2003; Kroeger et al., 2008) in tumors has been reported in multiple epithelial tumors and hematopoietic malignancies, indicating that promoter CpG methylation-mediated silencing is an important regulatory mechanism for disrupting cadherin members in tumorigenesis.
We have previously performed 1-Mb array comparative genomic hybridization (aCGH) analysis of carcinoma cell lines, and identified CDH11 as the only gene located at a 1-Mb hemizygous deletion detected at 16q22.1. We thus hypothesized that CDH11 could be a critical tumor suppressor gene implicated in tumorigenesis. Our present epigenetic and functional studies demonstrated that CDH11 was frequently inactivated by promoter methylation in multiple carcinomas and functioned as a tumor suppressor, by inducing tumor cell apoptosis and inhibiting cell motility and invasion, as well as cell stemness through Wnt/β-catenin and AKT/Rho A signaling.
Results
Identification of CDH11 as a candidate TSG at 16q21-22.1
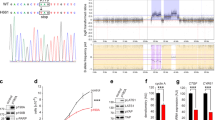
Genome-wide identification of gene deletions using aCGH identifies candidate TSG loci in tumors. Previously, we performed 1 Mb aCGH to identify DNA copy number aberrations in tumor cell lines including NPC (Ying et al., 2006), and identified an ∼1-Mb hemizygous deletion at 16q21-22.1 in three NPC cell lines (Figure 1a). Only one known gene—OB-cadherin (CDH11) is located at this deletion, indicating that CDH11 could be a candidate TSG for 16q21-22.1 deletion. We further assessed its expression in a series of human normal adult and fetal tissues using semiquantitative RT–PCR and detected its broad expression in normal tissues, though with variable expression levels (Figure 1b).
(a) Representative 1 Mb aCGH result showing a small hemizygous deletion including the CDH11 locus in NPC cell lines. Cytoband of 16q is shown. Normalized log2 signal intensity ratios from −1 to 1 are plotted. Each dark blue-colored dot represents a single BAC clone. Two BAC clones closest to the CDH11 locus (RP11-467L24 and RP11-229O3) are labeled with red dots and red rectangle frames. The CDH11 locus is shown in lower panel as in Ensemble Human Contig view (http://www.ensemble.org/). (b) CDH11 is broadly expressed in human normal adult tissues and fetal tissues, with GAPDH as a control. Sk.M., skeleton muscle; B.M., bone marrow; L.N., lymph node.
We then examined the expression levels of CDH11 in a series of tumor as well as immortalized but non-transformed normal epithelial cell lines. As shown in Figure 2b, significant reduction or silencing of CDH11 expression was frequently observed in multiple tumor cell lines of nasopharyngeal, esophageal, gastric, hepatocellular, colon, breast and cervix, infrequently in lung carcinoma cell lines (Supplementary Figure S1), but not in any of the normal cell lines. These results indicate that frequent downregulation of CDH11 is involved in multiple tumorigenesis.
(a) Schematic structure of the CDH11 CGI. Exon 1 (indicated with a black rectangle), CpG sites (short vertical lines), MSP sites, and BGS region analyzed are indicated. (b) CDH11 is frequently silenced and methylated in multiple carcinoma cell lines but expressed and unmethylated in immortalized but non-transformed epithelial cell lines (underlined). ESCC, esophageal carcinoma; GsCa, gastric carcinoma; CRC, colorectal cancer;M, methylated; U, unmethylated. (c) Validation of the specificity of MSP system for CDH11. No product was obtained for unbisulfited DNA. (d) Pharmacologic demethylation with Aza alone or combined with trichostatin A (A+T), or genetic demethylation in DKO cell line restored CDH11 expression in methylated/silenced tumor cell lines.
Frequent silencing of CDH11 by promoter CpG methylation in common carcinomas
Gene downregulation could result from either genetic or epigenetic mechanism. We checked whether CDH11 reduction could be due to genetic deletion. No homozygous deletion was detected in any silenced or downregulated cell line, as well as expressing normal epithelial cells (Supplementary Figure S2), suggesting that the downregulation/silencing of CDH11 is not due to genetic deletion, but rather epigenetic silencing.
Further analysis of CDH11 promoter showed that it contains a typical CpG island (http://ccnt.hsc.usc.edu/cpgislands2; Figure 2a), suggesting that CDH11 is likely subject to methylation-mediated silencing. We then analyzed CDH11 promoter methylation using a methylation-specific PCR (MSP) system, which was shown to be specific using DNA not bisulfite treated (Figure 2c). MSP analysis showed that CDH11 was frequently methylated in cell lines of nasopharyngeal, esophageal, gastric, hepatocellular, colorectal, breast and cervix carcinomas, well correlated with the expression levels (Figure 2b; Supplementary Figure S1). In contrast, no CDH11 methylation was found in any of the eight normal epithelial cell lines, suggesting that CDH11 methylation is tumor specific.
To examine the methylation status of CDH11 promoter in more detail, bisulfite genomic sequencing (BGS) analysis was performed with 44 CpG sites spanning the CDH11 core promoter and exon 1 in a 416-bp region analyzed. The BGS results confirmed the MSP data (Figures 3a and b). Thus, CDH11 silencing by DNA methylation is a widespread event in multiple tumors and might be one of the critical events involved in tumorigenesis.
Detailed BGS analyses of CDH11 promoter methylation. (a) NPC cell lines and immortalized normal cell line NP69. (b) Summarized percentage methylation of CDH11 in multiple carcinoma cell lines. (c) Pharmacologic and genetic demethylation of CDH11. The CDH11 transcription start site is shown as bent arrows. Circles, CpG sites analyzed; row of circles, an individual promoter allele that was cloned, randomly selected, and sequenced; filled circle, methylated CpG site; open circle, unmethylated CpG site.
Demethylation restored CDH11 expression and frequent methylation of CDH11 in multiple primary tumors
To test whether methylation directly mediates silencing of CDH11, several carcinoma cell lines with methylated and silenced CDH11 were treated with DNA methyltransferase inhibitor Aza, alone or combined with the HDAC inhibitor trichostatin A. Aza or/and trichostatin A restored the expression of CDH11 in these cell lines, accompanied by a decrease of methylated promoter alleles and an increase of unmethylated alleles, as analyzed by both MSP and BGS (Figures 2d and 3c). CDH11 could also be activated in colon cell line HCT116 by genetic disruption of both DNMT1 and DNMT3B, accompanied by CDH11 promoter demethylation, but not in single knockout (KO) of either DNA methyltransferase 1 (DNMT1) or DNMT3B (Figure 2d), similarly to other known and novel TSGs we previously studied, indicating that the maintenance of CDH11 promoter methylation is mediated by DNMT1 and DNMT3B.
CDH11 methylation was further detected in multiple primary carcinomas with variable frequencies, as summarized in Supplementary Table S2. CDH11 methylation was frequently detected in 94% (17 of 18) of NPC, 93% (13 of 14) of esophageal, 100% (13 of 13) of gastric, 66% (28 of 42) of HCC, 90% (10 of 11) of colonal and 91% (11 of 12) of breast carcinomas (Figure 4; Supplementary Table S2), while only seldom detected in normal tissues (nine normal nasopharyx, nine normal breast tissues, paired normal tissues of esophageal carcinoma and HCC; Figure 4). These results suggest that hypermethylation of the CDH11 promoter is a common event in multiple tumorigenesis.
CDH11 inhibited tumor cell clonogenicity and induced apoptosis
Immunostaining showed that CDH11 was primarily expressed in the cell membrane in CDH11-expressing HONE1 and KYSE150 cells, while no detectable expression of CDH11 was found in control cells (data not shown) (Supplementary Figure S3A). Confocal microscopy assay further demonstrated clear cell membrane localization of CDH11, with E-cadherin as a comparison, in CDH11-transfected HONE1 cells (Figure 5a). We also examined the expression of the two alternatively spliced forms of CDH11 protein. Western blot showed that the wild-type CDH11 (Mr∼110 kDa) was abundantly expressed in normal tissues including brain, testis, lung and trachea, as well as in CDH11-transfected tumor cells (Figures 5b and e), while no E-cadherin was detected in human testis as expected (Andersson et al., 1994; Conrad et al., 2008). In addition, a very weak band at Mr ∼85 kDa was also detected using CDH11 monoclonal antibody, but not with anti-Flag antibody, indicating that the wild-type CDH11 is the predominant form expressed in cells, thus serves as the major executor for its functions.
Subcellular localization and functional analyses of CDH11. (a) Confocal microscopy assay showed that CDH11 (red) is clearly expressed on the cell membrane in CDH11-transfected HONE1 cells using mouse anti-CDH11 monoclonal antibody, with double immunostaining for E-cadherin (green) as a control. DAPI counterstaining (blue) was used to visualize DNA. (b) CDH11 and E-cadherin protein expression detected in human normal tissues of brain, testis, lung and trachea by western blot. *Indicates truncated variant band. (c) CDH11 inhibits tumor cell growth. Representative colony formation assays. Quantitative analyses of colony numbers are shown in the right as values of mean±s.d., *P<0.05, **P<0.01. (d) CDH11 induces tumor cell apoptosis. TUNEL assays of CDH11 and vector-expressing tumor cells. *P<0.05. (e) Western blot showing upregulation of cleaved caspase 9, 7, 3 and cleaved poly (ADP-ribose) polymerase (PARP) in CDH11-expressing tumor cells.
The frequent downregulation and methylation of CDH11 in common tumors indicated that it is likely a tumor suppressor. Thus, we further explored the functional impact of CDH11 expression in tumor cells. Four cell lines (HONE1, KYSE150, CNE-2 and EC109) with complete methylation and silencing were selected. Results of colony formation assays showed that ectopic expression of CDH11 significantly suppressed the numbers of tumor cell colonies to <50% of the control cells (Figures 5c; Supplementary Figure S3B), indicating that CDH11 indeed suppresses the tumorigenecity of tumor cells.
We then checked whether CDH11 expression could induce tumor cell apoptosis. HONE1 and KYSE150 cells stably expressing CDH11 were tested using TUNEL (terminal nucleotidyltransferase-mediated nick end labeling) assay. Results showed that CDH11 expression significantly increased the number of apoptotic HONE1 and KYSE150 cells when compared with controls (from ∼1.5 to ∼10%; *P<0.05; Figure 5d). The data were confirmed by western blot for caspase-mediated cleavage of caspase 7, caspase 9, caspase 3 and poly (ADP-ribose) polymerase as apoptosis markers (Figure 5e), suggesting that CDH11 indeed induces apoptosis through the intrinsic caspase-dependent pathway.
CDH11 antagonized Wnt/β-catenin signaling pathway
To gain more insight of the molecular mechanisms underlying the tumor suppression by CDH11, we first examined whether CDH11 could counteract Wnt/β-catenin signaling. By TOPflash reporter assay measuring endogenous β-catenin/TcF activity, it was found that CDH11-expressing tumor cells showed 2–3-fold reduced TOPflash activities when compared with controls (Figure 6a), suggesting that CDH11 led to decreased β-catenin/TcF activities. Three genes as typical transcriptional targets of the Wnt/β-catenin/TcF signaling pathway, CCND1 (cyclin D1), c-Myc and MMP7 were thus evaluated. Results showed that CDH11 markedly repressed the promoter activities and expression of CCND1, c-Myc and MMP7 (Figures 6a and b).
(a) Inhibition of TcF activity and CCND1, c-Myc and MMP7 promoter reporter activities in CDH11-expressing tumor cells. *P<0.05. (b) Ectopic expression of CDH11 disrupted Wnt/β-catenin signaling pathway. Western blot was performed using antibodies against total β-catenin, active β-catenin, phospho-β-catenin (Ser552), phospho-GSK3β, CCND1, c-Myc and MMP7. α-Tubulin was used as a control. (c) CDH11 sequestered nuclear β-catenin to the cytoplasm. Endogenous β-catenin localization was visualized in CDH11- and vector-transfected HONE1 cells by indirect immunofluorescence. Original magnification, × 400.
β-Catenin, when enters the nucleus, has an important role as an activator of the transcription factor TcF/LEF. The expression and localization of the β-catenin were examined by western blot and immunostaining. No obvious change of total β-catenin protein levels was noted, however, levels of active- and phospho-β-catenin (ser552) that are the active forms dramatically decreased in CDH11-expressing tumor cells when compared with control cells, accompanied by downregulated GSK-3β phosphorylation (Figure 6b). In CDH11-transfected tumor cells, β-catenin translocated from nucleus to cytoplasm (Figure 6c), indicating that the extranuclear sequestration and inactivation of β-catenin mediated by CDH11 are responsible for reduced β-catenin/TcF-mediated transcriptional activity.
CDH11 regulated the organization of actin cytoskeleton, cell migration and invasion
Classical cadherins are directly or indirectly linked to actin cytoskeleton organization. The impact of CDH11 expression on actin filament integrity was investigated by immunofluorescence. As shown in Figure 7a, the formation of actin stress fibers was disassembled in CDH11-expressing cells but not in control cells. Furthermore, downregulation of phospho-Rho A and AKT, as well as upregulation of phospho-JNK was found in CDH11-expressing cells by western blot (Figure 7b), consistent with their roles in impairing stress fibers.
(a) Impaired actin stress fiber organization in CDH11-expressing HONE1 cells. Original magnification, × 400. (b) Expression of several key regulators of actin stress fiber reorganization in CDH11- and vector-transfected cells. Western blot analysis was done using antibodies against phospho-Akt, phospho-JNK, phospho-Rho A and total Rho A. (c) Wound-healing assay of tumor cells transfected with either vector or CDH11. Pictures were taken at 0, 12, 24 or 40 h. Right panel: width of remaining open wound measured in relation to time 0 h separation. (d) CDH11 inhibited the invasive activity of tumor cells. Original magnification, × 400. *P<0.05.
As remodeling of actin cytoskeleton is critical for changes in cell shape, migration and invasion, the effect of CDH11 on tumor cell migration and invasion was further explored. Scratch wound-healing assays showed that CDH11-expressing cells were less proficient in closing an artificial wound than the vector-transfected cells on a confluent monolayer (Figure 7c, *P<0.05). Moreover, CDH11-expressing cells displayed significantly suppressed invasiveness compared with controls in matrigel invasion assays (Figure 7d, *P<0.05). Collectively, these results indicate that CDH11 expression could inhibit tumor cell metastasis and migration via disrupting stress filament integrity mediated by multiple signaling pathways.
CDH11 regulated epithelial–mesenchymal transition and stemness
Epithelial–mesenchymal transition (EMT) has a critical role in tumor cell metastasis by reducing cell–cell contact and increased motility. The influence of CDH11 expression on EMT regulation in tumor cells was further assessed. Dramatic morphological changes were observed in CDH11-expressing tumor cells, in which spindle-like, fibroblastic morphology was replaced by the typical cobblestone-like appearance of normal epithelial cells (Figure 8a), indicating that CDH11 most likely reverses tumor cell EMT. Epithelial and mesenchymal markers were examined to determine whether CDH11 negatively regulates EMT. CDH11-expressing cells exhibited a reversed EMT phenotype, including upregulated epithelial marker E-cadherin and downregulated mesenchymal marker Vimentin (Figure 8c), which was further confirmed by immunofluorescence (Figure 8b).
(a) Morphology changes of HONE1 cells transfected with CDH11 or empty vector by phase-contrast microscopy. Original magnification, × 400. (b) Indirect immunofluorescence detecting the expression of E-cadherin and Vimentin in CDH11- or vector-transfected HONE1 cells. (c) Western blot showing the expression of E-cadherin and Vimentin in CDH11- or vector-transfected cells. α-Tubulin was used as a loading control. (d) Downregulation of representative stem cell markers in CDH11-transfected tumor cells. *Indicates significantly downregulated bands.
As EMT is also linked to epithelial stem cell properties, we further examined whether CDH11 could inhibit the tumor cell stemness. Representative stem cell markers were analyzed by RT–PCR. CDH11 expression downregulated 6 out of 7 stem cell markers: NANOG, DPPA5, ABCG2, c-Myc, MAD2 and OCT4, at the transcriptional level, except for KLF4 (Figure 8d). Taken together, these results suggest that CDH11 inhibits both EMT and stemness of carcinoma cells.
Discussion
In this study, we found that although CDH11 is ubiquitously expressed in human normal adult and fetal tissues, it is frequently methylated and silenced in multiple common carcinoma cell lines and primary tumors but seldom in normal tissues. Pharmacologic or genetic demethylation resulted in the demethylation and restoration of CDH11 expression. We also found that CDH11 functions as a pro-apoptotic tumor suppressor, through antagonizing Wnt/β-catenin and AKT/Rho A signaling and disrupting stress fiber formation, and further inhibits the EMT and stemness of tumor cells and their migration and invasion. Our study appears to be the first study demonstrating the functional mechanisms of CDH11, and strongly supports the notion that CDH11 is a tumor suppressor for multiple carcinomas.
CDH11, one of the type II classical cadherins, was originally identified in osteoblasts (Okazaki et al., 1994), subsequently found overexpressed in certain invasive tumors (Pishvaian et al., 1999; Chu et al., 2008; Huang et al., 2010). Earlier studies suggested that CDH11 was a candidate oncogene for some tumor types like prostate cancer (Chu et al., 2008; Huang et al., 2010), breast cancer (Pishvaian et al., 1999) and oral cancer (Choi et al., 2008). Recently, increasing evidence indicate that CDH11 might be a candidate tumor suppressor for locus 16q, a frequent loss of heterozygosity region in multiple cancers. For example, genetic loss of CDH11 has been identified in more than half of retinoblastoma, implicating CDH11 as a potential TSG in retinoblastoma (Marchong et al., 2004). Using 16q-specific aCGH, CDH11 was identified to be a candidate tumor suppressor in breast tumorigenesis (Roylance et al., 2006). Integrative epigenomic and genomic analysis of malignant pheochromocytoma also confirmed the potential of CDH11 as a candidate tumor suppressor (Sandgren et al., 2010). CDH11 overexpression suppressed pulmonary metastasis of osteosarcoma in vivo (Nakajima et al., 2008). These results are in line with ours and support the view that CDH11 is a potent tumor suppressor for certain tumors.
TSGs are more frequently inactivated through epigenetic mechanisms such as promoter CpG methylation, or a combination of genetic and epigenetic inactivation, than biallelic genetic inactivation alone (Jones and Baylin, 2007). Three cadherin members (CDH1 and CDH13 at 16q, CDH4; Miotto et al., 2004) as TSGs being inactivated genetically and/or epigenetically in various tumors and involved in tumor cell invasion and metastasis have been previously reported. We reported here that CDH11 is the fourth cadherin member functioning as a TSG in multiple tumors, but silenced by promoter CpG methylation in multiple carcinomas. We also found that no methylation was detected in few carcinoma cell lines with reduced CDH11 expression, suggesting that histone modification may be an alternative mechanism for CDH11 downregulation in tumorigenesis.
Cdh11 was first identified to act as a candidate tumor suppressor in a murine retinoblastoma model by facilitating tumor cell death (Marchong et al., 2010), but how it functions in human tumorigenesis is not well understood. CDH11 is unique among cadherins as two alternatively spliced forms are expressed simultaneously in cells: the intact form and a COOH terminus-truncated variant lacking homophilic cell–cell adhesion ability thus as a dominant-negative product which is also the secreted form (Kashima et al., 1999; Pishvaian et al., 1999). We found that when the full-length CDH11 was introduced into tumor cells, its intact form (∼110 kDa) is the predominant isoform expressed, although a tiny amount of its truncated variant was also detected, in line with previous studies (Kashima et al., 1999; Pishvaian et al., 1999; Feltes et al., 2002). Subcellular localization of CDH11 in CDH11-expressing tumor cells exhibited the clear membrane-localizing feature of cadherin family members. Furthermore, we identified that the intact form CDH11 significantly suppressed the malignant properties of transfected cells including proliferation, migration/invasion and promoted tumor cell apoptosis, acting as a functional tumor suppressor, in line with the previous finding that intact CDH11 inhibits the invasion of breast cancer cells (Pishvaian et al., 1999; Feltes et al., 2002). While an invasion-promoting effect of its truncated variant has been reported (Feltes et al., 2002), more studies are needed to delineate the different role of the truncated variant of CDH11 in tumorigenesis.
Cadherin-associated β-catenin pool having a distinct role from its nuclear transcription role (Berx and van Roy, 2009). As a functional adherens junction molecule, CDH11 mediates calcium-dependent cell–cell adhesion via recruiting α-catenin, β-catenin and p120ctn to the membrane, involved in the regulation of Wnt/β-catenin signaling pathway (Feltes et al., 2002; Huang et al., 2010). In our study, CDH11 was found to facilitate extranuclear sequestration of β-catenin, and reduce β-catenin/TcF-mediated proliferation and transcriptional activity, thus disrupting Wnt/β-catenin signaling, as well as cross-talking with Rho A, AKT and JNK signaling. Consistent with this mechanism, we observed disrupted actin polymerization and reversed EMT phenotypes after CDH11 expression. EMT promotes the production of cells with property of self-renewing stem cells, thus facilitating the execution of the invasion-metastasis cascade (Mani et al., 2008; Giannoni et al., 2010). The role of cadherin family members on cell stemness is complicated, depending on coordinately regulated cadherins (Hosokawa et al., 2010). E-cadherin has been identified to be a critical molecule for embryonic stem cell pluripotency (Karpowicz et al., 2009; Chen et al., 2010; Redmer et al., 2011). In our study, the downregulation of several stem cell markers by CDH11 suggests the potential of CDH11 in modulating tumor cell stemness, which may be due to increased E-cadherin and further link to its tumor suppressive role.
In summary, our study identifies CDH11 as a functional tumor suppressor and an important regulator of Wnt/β-catenin and AKT/Rho A signaling, with frequent epigenetic inactivation in common carcinomas. Our study further extends the current understanding on the role of cadherin family in tumorigenesis.
Materials and methods
Cell lines and tissue samples
A series of tumor cell lines were used (Wang et al., 2009; Cheng et al., 2010). Immortalized, non-transformed normal epithelial cell lines (NP69, Het-1A, NE083, NE1, NE3, CCD841-CoN, HMEC and HMEpC) were used as normal controls. HCT116 cell lines with genetic KO of DNMTs: HCT116 DNMT1−/− (1KO), HCT116 DNMT3B−/− (3BKO) and HCT116 DNMT1−/− DNMT3B−/− (DKO) cells (gifts of Bert Vogelstein, Johns Hopkins University, Baltimore) were also used. Cell lines were obtained either from American Type Culture Collection or from collaborators. Cell lines were treated with 10 μmol/L 5-aza-2′-deoxycytidine (Aza, Sigma-Aldrich, St Louis, MO, USA) for 3 days or further treated with 100 nmol/L trichostatin A (Cayman Chemical Co., Ann Arbor, MI, USA) for additional ∼16 h as described previously (Qiu et al., 2004; Ying et al., 2006). Normal adult and fetal tissue RNA and protein samples were purchased commercially (Stratagene, La Jolla, CA, USA or Millipore-Chemicon, Billerica, MA, USA). DNA samples of normal nasopharyngeal and breast tissues, Asian Chinese NPC, paired Hong Kong Chinese esophageal carcinomas (T) and the matching surgical marginal normal tissues (N), and other carcinomas have been described previously (Cui et al., 2008; Cheng et al., 2010).
Antibodies
Antibodies used were cleaved caspase-7 (9491), cleaved caspase-9 (9505), cleaved caspase-3 (9661), cleaved poly (ADP-ribose) polymerase (9541), phospho-β-Catenin (Ser552), phospho-GSK-3β (Ser9), phospho-AKT (Ser473), phospho-SAPK/JNK (Thr183/Tyr185), RhoA (2117) and E-cadherin (4065) (Cell Signaling, Beverly, MA, USA); Flag M2 (F3165), Vimentin (V6630) (Sigma-Aldrich) and active β-catenin (anti-ABC, 05-665, Upstate, Lake Placid, NY, USA); total β-catenin (M3539), anti-mouse Ig G-HRP (P0161), anti-rabbit Ig G-HRP (P0448) (Dako, Glostrup, Denmark); CCND1 (sc-20044), c-myc (sc-764), phospho-RhoA (Ser188) (sc-32954) (Santa Cruz, CA, USA); MMP7 (MS-813-P0, Thermo Scientific, Fremont, CA, USA); monoclonal CDH 11 antibody (32-1700) (Invitrogen, Carlsbad, CA, USA); α-tubulin (Lab Vision Corporation, Fremont, CA, USA).
Semiquantitative RT–PCR
Semiquantitative RT–PCR was performed as described before (Jin et al., 2007). GAPDH was amplified as a control. Primers used in this study are listed in Supplementary Table S1.
Deletion analysis of CDH11 by multiplex PCR
Homozygous deletion of CDH11 was examined using multiplex genomic DNA PCR as previously described (Qiu et al., 2004; Cui et al., 2008). Primer sequences are shown in Supplementary Table S1.
Bisulfite treatment and promoter methylation analysis
Bisulfite modification of DNA, MSP and BGS were carried out as described previously (Tao et al., 1999, 2002). MSP and BGS primers are listed in Supplementary Table S1.
Construction of CDH11-expressing vector and generation of stable cell lines
Full-length cDNA of CDH11 was PCR cloned from human trachea RNA (BD Clontech, Palo Alto, CA, USA) with the primers (Supplementary Table 1) and sequence verified. pcDNA3.1(+)-Flag-CDH11 plasmid was then constructed as previously (Cheng et al., 2010; Li et al., 2010). This expression construct was transfected into HONE1 and KYSE150 cells using Lipofectamine 2000 (Invitrogen). The cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum and selected in 400 μg/ml of G418 for 20–30 days to establish stable cell pools.
Colony formation assay
Colony formation assays were carried out as previously described (Jin et al., 2007; Ying et al., 2008). Briefly, cells were cultured overnight in a 12-well plate 1 (1.0 × 105 per well) and transfected with empty vector or CDH11-expressing plasmid using Lipofectamine 2000 (Invitrogen). Forty-eight hours later, the transfectants were replated in triplicate and cultured for 10–15 days in complete medium containing G418 (400 μg/ml). Surviving colonies were stained with gentian violet after methanol fixation and visible colonies (⩾50 cells) were counted.
TUNEL assay
Cells cultured on coverslips were fixed with 4% paraformaldehyde in phosphate-buffered saline for 15 min at room temperature and permeabilized with 0.1% Triton X-100 in phosphate-buffered saline for 2 min on ice. TUNEL staining was done using the In Situ Cell Death Detection Kit (Roche, Mannheim, Germany) according to the manufacturer's instruction.
Western blot
Cell lysates were prepared by incubating the cell pellets in lysis buffer (50 mmol/l Tris–HCl, pH 8.0; 150 mmol/L NaCl, 0.5% NP-40) for 30 min on ice, followed by centrifugation at 14 000 × g for 15 min at 4 °C. For western blot, membranes were incubated with primary antibodies for 1 h at room temperature or overnight at 4 °C followed by incubation with a secondary antibody. Immunoreactive bands were visualized using Western blot Luminol reagent (GE Healthcare Bio-Sciences, Piscataway, NJ, USA) according to the manufacturer's protocol.
Dual-luciferase reporter assay
TcF transcriptional activity and its target genes promoter activities were determined by luciferase reporter assays. The TcF-responsive luciferase construct pTopFlash was kindly provided by Prof. Christof Niehrs (German Cancer Research Center, DKFZ). CCND1-, c-Myc- and MMP7-promoter reporter constructs containing TcF-responsive element were cloned in our laboratory. Cells were transiently transfected in triplicate with one of the luciferase reporters and phRL-TK (Promega, Madison, WI, USA). After 48 h of transfection, luciferase activities were determined using a dual-luciferase reporter assay kit (Promega). Relative luciferase activities were determined and normalized using Renilla reniformis luciferase activity as an internal control.
Indirect immunofluorescence
Cells grown on coverslips were stained by indirect immunofluorescence as described previously (Hu et al., 2009). Briefly, cells were incubated with primary antibodies against CDH11, β-catenin, E-cadherin, or Vimentin and then incubated with Alexa Fluor 594- (Invitrogen Molecular Probes, Carlsbad, CA, USA) or FITC-conjugated (Dako) secondary antibody against mouse or rabbit IgG. To analyze the effects of CDH11 on actin stress fiber formation, cells were serum starved for 24 h before incubation in serum-containing 10% fetal bovine serum medium. After 1 h, cells were fixed and stained by Rhodamine-labeled phalloidin (Invitrogen Molecular Probes). Cells were then counterstained with DAPI and imaged with an Olympus BX51 microscope (Olympus Corporation, Tokyo, Japan) and Leica TCS SP5 confocal microscope (Leica Microsystems CMS GmbH, Mannheim, Germany). Confocal images of 2048 × 2048 pixels were acquired and assembled.
Wound-healing assay and matrigel invasion assays
Cell motility was assessed using a scratch wound-healing assay (Ying et al., 2008). Stably transfected cells were cultured in 6-well plates until confluent. The cell layers were carefully wounded using sterile tips and washed twice with fresh medium. After incubation for 12, 24 and 40 h, the cells were photographed under a phase-contrast microscope. The experiments were performed in triplicate.
In-vitro invasion assays were carried out in BD BioCoat Matrigel chambers (Transwell, BD Biosciences, Heidelberg, Germany) as described previously (Hu et al., 2009).
Statistical analysis
Results are shown as values of mean±s.d. Statistical analyses were performed using the Student's t-test to determine P-values.
References
Andersson AM, Edvardsen K, Skakkebaek NE . (1994). Expression and localization of N- and E-cadherin in the human testis and epididymis. Int J Androl 17: 174–180.
Andreeva AV, Kutuzov MA . (2010). Cadherin 13 in cancer. Genes Chromosomes Cancer 49: 775–790.
Angst BD, Marcozzi C, Magee AI . (2001). The cadherin superfamily: diversity in form and function. J Cell Sci 114: 629–641.
Aqeilan RI, Trapasso F, Hussain S, Costinean S, Marshall D, Pekarsky Y et al. (2007). Targeted deletion of Wwox reveals a tumor suppressor function. Proc Natl Acad Sci USA 104: 3949–3954.
Berx G, van Roy F . (2009). Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol 1: a003129.
Bird A . (2002). DNA methylation patterns and epigenetic memory. Genes Dev 16: 6–21.
Callen DF, Crawford J, Derwas C, Cleton-Jansen AM, Cornelisse CJ, Baker E . (2002). Defining regions of loss of heterozygosity of 16q in breast cancer cell lines. Cancer Genet Cytogenet 133: 76–82.
Chen T, Yuan D, Wei B, Jiang J, Kang J, Ling K et al. (2010). E-cadherin-mediated cell-cell contact is critical for induced pluripotent stem cell generation. Stem Cells 28: 1315–1325.
Cheng Y, Geng H, Cheng SH, Liang P, Bai Y, Li J et al. (2010). KRAB zinc finger protein ZNF382 is a proapoptotic tumor suppressor that represses multiple oncogenes and is commonly silenced in multiple carcinomas. Cancer Res 70: 6516–6526.
Choi P, Jordan CD, Mendez E, Houck J, Yueh B, Farwell DG et al. (2008). Examination of oral cancer biomarkers by tissue microarray analysis. Arch Otolaryngol Head Neck Surg 134: 539–546.
Chu K, Cheng CJ, Ye X, Lee YC, Zurita AJ, Chen DT et al. (2008). Cadherin-11 promotes the metastasis of prostate cancer cells to bone. Mol Cancer Res 6: 1259–1267.
Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M et al. (2008). Generation of pluripotent stem cells from adult human testis. Nature 456: 344–349.
Cui Y, Ying Y, van Hasselt A, Ng KM, Yu J, Zhang Q et al. (2008). OPCML is a broad tumor suppressor for multiple carcinomas and lymphomas with frequently epigenetic inactivation. PLoS One 3: e2990.
Eads CA, Lord RV, Wickramasinghe K, Long TI, Kurumboor SK, Bernstein L et al. (2001). Epigenetic patterns in the progression of esophageal adenocarcinoma. Cancer Res 61: 3410–3418.
Feltes CM, Kudo A, Blaschuk O, Byers SW . (2002). An alternatively spliced cadherin-11 enhances human breast cancer cell invasion. Cancer Res 62: 6688–6697.
Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L et al. (2010). Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res 70: 6945–6956.
Hiraguri S, Godfrey T, Nakamura H, Graff J, Collins C, Shayesteh L et al. (1998). Mechanisms of inactivation of E-cadherin in breast cancer cell lines. Cancer Res 58: 1972–1977.
Hosokawa K, Arai F, Yoshihara H, Iwasaki H, Hembree M, Yin T et al. (2010). Cadherin-based adhesion is a potential target for niche manipulation to protect hematopoietic stem cells in adult bone marrow. Cell Stem Cell 6: 194–198.
Hu XT, Zhang FB, Fan YC, Shu XS, Wong AH, Zhou W et al. (2009). Phospholipase C delta 1 is a novel 3p22.3 tumor suppressor involved in cytoskeleton organization, with its epigenetic silencing correlated with high-stage gastric cancer. Oncogene 28: 2466–2475.
Huang CF, Lira C, Chu K, Bilen MA, Lee YC, Ye X et al. (2010). Cadherin-11 increases migration and invasion of prostate cancer cells and enhances their interaction with osteoblasts. Cancer Res 70: 4580–4589.
Hui AB, Lo KW, Leung SF, Teo P, Fung MK, To KF et al. (1999). Detection of recurrent chromosomal gains and losses in primary nasopharyngeal carcinoma by comparative genomic hybridisation. Int J Cancer 82: 498–503.
Jin H, Wang X, Ying J, Wong AH, Cui Y, Srivastava G et al. (2007). Epigenetic silencing of a Ca(2+)-regulated Ras GTPase-activating protein RASAL defines a new mechanism of Ras activation in human cancers. Proc Natl Acad Sci USA 104: 12353–12358.
Jones PA, Baylin SB . (2007). The epigenomics of cancer. Cell 128: 683–692.
Karpowicz P, Willaime-Morawek S, Balenci L, DeVeale B, Inoue T, van der Kooy D . (2009). E-Cadherin regulates neural stem cell self-renewal. J Neurosci 29: 3885–3896.
Kashima T, Kawaguchi J, Takeshita S, Kuroda M, Takanashi M, Horiuchi H et al. (1999). Anomalous cadherin expression in osteosarcoma. Possible relationships to metastasis and morphogenesis. Am J Pathol 155: 1549–1555.
Kochetkova M, McKenzie OL, Bais AJ, Martin JM, Secker GA, Seshadri R et al. (2002). CBFA2T3 (MTG16) is a putative breast tumor suppressor gene from the breast cancer loss of heterozygosity region at 16q24.3. Cancer Res 62: 4599–4604.
Kremmidiotis G, Baker E, Crawford J, Eyre HJ, Nahmias J, Callen DF . (1998). Localization of human cadherin genes to chromosome regions exhibiting cancer-related loss of heterozygosity. Genomics 49: 467–471.
Kroeger H, Jelinek J, Estecio MR, He R, Kondo K, Chung W et al. (2008). Aberrant CpG island methylation in acute myeloid leukemia is accentuated at relapse. Blood 112: 1366–1373.
Li L, Tao Q, Jin H, van Hasselt A, Poon FF, Wang X et al. (2010). The tumor suppressor UCHL1 forms a complex with p53/MDM2/ARF to promote p53 signaling and is frequently silenced in nasopharyngeal carcinoma. Clin Cancer Res 16: 2949–2958.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133: 704–715.
Marchong MN, Chen D, Corson TW, Lee C, Harmandayan M, Bowles E et al. (2004). Minimal 16q genomic loss implicates cadherin-11 in retinoblastoma. Mol Cancer Res 2: 495–503.
Marchong MN, Yurkowski C, Ma C, Spencer C, Pajovic S, Gallie BL . (2010). Cdh11 acts as a tumor suppressor in a murine retinoblastoma model by facilitating tumor cell death. PLoS Genet 6: e1000923.
Margulis A, Zhang W, Alt-Holland A, Crawford HC, Fusenig NE, Garlick JA . (2005). E-cadherin suppression accelerates squamous cell carcinoma progression in three-dimensional, human tissue constructs. Cancer Res 65: 1783–1791.
Miotto E, Sabbioni S, Veronese A, Calin GA, Gullini S, Liboni A et al. (2004). Frequent aberrant methylation of the CDH4 gene promoter in human colorectal and gastric cancer. Cancer Res 64: 8156–8159.
Nakajima G, Patino-Garcia A, Bruheim S, Xi Y, San Julian M, Lecanda F et al. (2008). CDH11 expression is associated with survival in patients with osteosarcoma. Cancer Genomics Proteomics 5: 37–42.
Nishioka N, Yashiro M, Inoue T, Matsuoka T, Ohira M, Chung KH . (2001). A candidate tumor suppressor locus for scirrhous gastric cancer at chromosome 18q 12.2. Int J Oncol 18: 317–322.
Okazaki M, Takeshita S, Kawai S, Kikuno R, Tsujimura A, Kudo A et al. (1994). Molecular cloning and characterization of OB-cadherin, a new member of cadherin family expressed in osteoblasts. J Biol Chem 269: 12092–12098.
Pishvaian MJ, Feltes CM, Thompson P, Bussemakers MJ, Schalken JA, Byers SW . (1999). Cadherin-11 is expressed in invasive breast cancer cell lines. Cancer Res 59: 947–952.
Qiu GH, Tan LK, Loh KS, Lim CY, Srivastava G, Tsai ST et al. (2004). The candidate tumor suppressor gene BLU, located at the commonly deleted region 3p21.3, is an E2F-regulated, stress-responsive gene and inactivated by both epigenetic and genetic mechanisms in nasopharyngeal carcinoma. Oncogene 23: 4793–4806.
Redmer T, Diecke S, Grigoryan T, Quiroga-Negreira A, Birchmeier W, Besser D . (2011). E-cadherin is crucial for embryonic stem cell pluripotency and can replace OCT4 during somatic cell reprogramming. EMBO Rep 12: 720–726.
Roman-Gomez J, Castillejo JA, Jimenez A, Cervantes F, Boque C, Hermosin L et al. (2003). Cadherin-13, a mediator of calcium-dependent cell-cell adhesion, is silenced by methylation in chronic myeloid leukemia and correlates with pretreatment risk profile and cytogenetic response to interferon alfa. J Clin Oncol 21: 1472–1479.
Roylance R, Gorman P, Papior T, Wan YL, Ives M, Watson JE et al. (2006). A comprehensive study of chromosome 16q in invasive ductal and lobular breast carcinoma using array CGH. Oncogene 25: 6544–6553.
Sandgren J, Andersson R, Rada-Iglesias A, Enroth S, Akerstrom G, Dumanski JP et al. (2010). Integrative epigenomic and genomic analysis of malignant pheochromocytoma. Exp Mol Med 42: 484–502.
Stanton SE, Shin SW, Johnson BE, Meyerson M . (2000). Recurrent allelic deletions of chromosome arms 15q and 16q in human small cell lung carcinomas. Genes Chromosomes Cancer 27: 323–331.
Sun X, Frierson HF, Chen C, Li C, Ran Q, Otto KB et al. (2005). Frequent somatic mutations of the transcription factor ATBF1 in human prostate cancer. Nat Genet 37: 407–412.
Suzuki H, Komiya A, Emi M, Kuramochi H, Shiraishi T, Yatani R et al. (1996). Three distinct commonly deleted regions of chromosome arm 16q in human primary and metastatic prostate cancers. Genes Chromosomes Cancer 17: 225–233.
Tao Q, Huang H, Geiman TM, Lim CY, Fu L, Qiu GH et al. (2002). Defective de novo methylation of viral and cellular DNA sequences in ICF syndrome cells. Hum Mol Genet 11: 2091–2102.
Tao Q, Swinnen LJ, Yang J, Srivastava G, Robertson KD, Ambinder RF . (1999). Methylation status of the Epstein-Barr virus major latent promoter C in iatrogenic B cell lymphoproliferative disease. Application of PCR-based analysis. Am J Pathol 155: 619–625.
Toyooka KO, Toyooka S, Virmani AK, Sathyanarayana UG, Euhus DM, Gilcrease M et al. (2001). Loss of expression and aberrant methylation of the CDH13 (H-cadherin) gene in breast and lung carcinomas. Cancer Res 61: 4556–4560.
Toyooka S, Toyooka KO, Harada K, Miyajima K, Makarla P, Sathyanarayana UG et al. (2002). Aberrant methylation of the CDH13 (H-cadherin) promoter region in colorectal cancers and adenomas. Cancer Res 62: 3382–3386.
Wang Y, Li J, Cui Y, Li T, Ng KM, Geng H et al. (2009). CMTM3, located at the critical tumor suppressor locus 16q22.1, is silenced by CpG methylation in carcinomas and inhibits tumor cell growth through inducing apoptosis. Cancer Res 69: 5194–5201.
Wheeler JM, Kim HC, Efstathiou JA, Ilyas M, Mortensen NJ, Bodmer WF . (2001). Hypermethylation of the promoter region of the E-cadherin gene (CDH1) in sporadic and ulcerative colitis associated colorectal cancer. Gut 48: 367–371.
Yagi T, Takeichi M . (2000). Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev 14: 1169–1180.
Yeh SH, Chen PJ, Lai MY, Chen DS . (1996). Allelic loss on chromosomes 4q and 16q in hepatocellular carcinoma: association with elevated alpha-fetoprotein production. Gastroenterology 110: 184–192.
Ying J, Li H, Seng TJ, Langford C, Srivastava G, Tsao SW et al. (2006). Functional epigenetics identifies a protocadherin PCDH10 as a candidate tumor suppressor for nasopharyngeal, esophageal and multiple other carcinomas with frequent methylation. Oncogene 25: 1070–1080.
Ying J, Li H, Yu J, Ng KM, Poon FF, Wong SC et al. (2008). WNT5A exhibits tumor-suppressive activity through antagonizing the Wnt/beta-catenin signaling, and is frequently methylated in colorectal cancer. Clin Cancer Res 14: 55–61.
Acknowledgements
We thank Drs Tzer Jing Seng and Cordelia Langford for their help of array-CGH and Dr Colin D MacCalman for providing the CDH11 cDNA. This study was supported by grants from Hong Kong RGC (GRF #473908), National Basic Research Program of China (2011CB504305) and National Natural Science Foundation (No. 81172582).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Li, L., Ying, J., Li, H. et al. The human cadherin 11 is a pro-apoptotic tumor suppressor modulating cell stemness through Wnt/β-catenin signaling and silenced in common carcinomas. Oncogene 31, 3901–3912 (2012). https://doi.org/10.1038/onc.2011.541
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.541
Keywords
This article is cited by
-
Pathogenic variants in CDH11 impair cell adhesion and cause Teebi hypertelorism syndrome
Human Genetics (2021)
-
MFAP2 is overexpressed in gastric cancer and promotes motility via the MFAP2/integrin α5β1/FAK/ERK pathway
Oncogenesis (2020)
-
Targeting cancer stem cell pathways for cancer therapy
Signal Transduction and Targeted Therapy (2020)
-
CpG Islands Methylation Analysis of CDH11, EphA5, and HS3ST2 Genes in Gastric Adenocarcinoma Patients
Journal of Gastrointestinal Cancer (2020)
-
RETRACTED ARTICLE: A positive feedback loop consisting of C12orf59/NF-κB/CDH11 promotes gastric cancer invasion and metastasis
Journal of Experimental & Clinical Cancer Research (2019)