Abstract
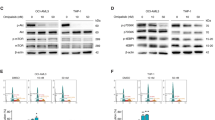
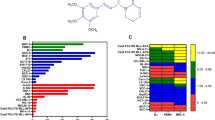
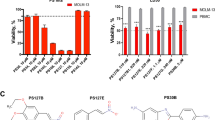
Despite recent progress in the treatment of acute myeloid leukemia (AML), the prognosis of this rather heterogeneous disease remains poor and novel chemotherapeutics that specifically target leukemic cells must be developed. To address this need at the preclinical level, we implemented a high content imaging-based screen for the identification of small agents that induce AML cell death in vitro. Among a panel of 1040 Food and Drug Administration-approved agents, we identified pyrithione zinc (PZ) and ouabain (OUA) as potential antileukemic compounds. Both PZ and OUA efficiently induced cell death associated with apoptotic chromatin condensation and inhibition of nuclear factor-κB survival signaling, leading to reduced expression of antiapoptotic proteins, in several AML cell lines. PZ- and OUA-induced cell death was associated with the permeabilization of the outer mitochondrial membrane and led to the release of cytochrome c followed by caspase activation. Both PZ and OUA exerted significant anticancer effects in vivo, on human AML cells xenografts as well as ex vivo, on CD34+ (but not CD34−) malignant myeloblasts from AML patients. Altogether, our results suggest that PZ and OUA may exhibit antileukemic effects by inducing the apoptotic demise of AML cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bielawski K, Winnicka K, Bielawska A . (2006). Inhibition of DNA topoisomerases I and II, and growth inhibition of breast cancer MCF-7 cells by ouabain, digoxin and proscillaridin A. Biol Pharm Bull 29: 1493–1497.
Boehrer S, Ades L, Braun T, Galluzzi L, Grosjean J, Fabre C et al. (2008). Erlotinib exhibits antineoplastic off-target effects in AML and MDS: a preclinical study. Blood 111: 2170–2180.
Burnett A, Wetzler M, Lowenberg B . (2011). Therapeutic advances in acute myeloid leukemia. J Clin Oncol 29: 487–494.
Castedo M, Ferri K, Roumier T, Metivier D, Zamzami N, Kroemer G . (2002). Quantitation of mitochondrial alterations associated with apoptosis. J Immunol Methods 265: 39–47.
Chan G, Pilichowska M . (2007). Complete remission in a patient with acute myelogenous leukemia treated with erlotinib for non small-cell lung cancer. Blood 110: 1079–1080.
de La Motte Rouge T, Galluzzi L, Olaussen KA, Zermati Y, Tasdemir E, Robert T et al. (2007). A novel epidermal growth factor receptor inhibitor promotes apoptosis in non-small cell lung cancer cells resistant to erlotinib. Cancer Res 67: 6253–6262.
Edwards BS, Young SM, Ivnitsky-Steele I, Ye RD, Prossnitz ER, Sklar LA . (2009). High-content screening: flow cytometry analysis. Methods Mol Biol 486: 151–165.
Galluzzi L, Aaronson SA, Abrams J, Alnemri ES, Andrews DW, Baehrecke EH et al. (2009). Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ 16: 1093–1107.
Galluzzi L, Zamzami N, de La Motte Rouge T, Lemaire C, Brenner C, Kroemer G . (2007). Methods for the assessment of mitochondrial membrane permeabilization in apoptosis. Apoptosis 12: 803–813.
Hahn CK, Berchuck JE, Ross KN, Kakoza RM, Clauser K, Schinzel AC et al. (2009). Proteomic and genetic approaches identify Syk as an AML target. Cancer Cell 16: 281–294.
Hallbook H, Felth J, Eriksson A, Fryknas M, Bohlin L, Larsson R et al. (2011). Ex vivo activity of cardiac glycosides in acute leukaemia. PLoS One 6: e15718.
Hoffmann J, Vitale I, Buchmann B, Galluzzi L, Schwede W, Senovilla L et al. (2008). Improved cellular pharmacokinetics and pharmacodynamics underlie the wide anticancer activity of sagopilone. Cancer Res 68: 5301–5308.
Juncker T, Cerella C, Teiten MH, Morceau F, Schumacher M, Ghelfi J et al. (2011). UNBS1450, a steroid cardiac glycoside inducing apoptotic cell death in human leukemia cells. Biochem Pharmacol 81: 13–23.
Kepp O, Galluzzi L, Lipinski M, Yuan J, Kroemer G . (2011). Cell death assays for drug discovery. Nat Rev Drug Discov 10: 221–237.
Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS et al. (2008). Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4: 151–175.
Kometiani P, Liu L, Askari A . (2005). Digitalis-induced signaling by Na+/K+-ATPase in human breast cancer cells. Mol Pharmacol 67: 929–936.
Lamore SD, Cabello CM, Wondrak GT . (2009). The topical antimicrobial zinc pyrithione is a heat shock response inducer that causes DNA damage and PARP-dependent energy crisis in human skin cells. Cell Stress Chaperones 15: 309–322.
Lamore SD, Wondrak GT . (2011). Zinc pyrithione impairs zinc homeostasis and upregulates stress response gene expression in reconstructed human epidermis. Biometals 24: 875–890.
Lopez-Lazaro M . (2007). Digitoxin as an anticancer agent with selectivity for cancer cells: possible mechanisms involved. Expert Opin Ther Targets 11: 1043–1053.
Metivier D, Dallaporta B, Zamzami N, Larochette N, Susin SA, Marzo I et al. (1998). Cytofluorometric detection of mitochondrial alterations in early CD95/Fas/APO-1-triggered apoptosis of Jurkat T lymphoma cells. Comparison of seven mitochondrion-specific fluorochromes. Immunol Lett 61: 157–163.
Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D et al. (1991). Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 83: 757–766.
Newman RA, Yang P, Hittelman WN, Lu T, Ho DH, Ni D et al. (2006). Oleandrin-mediated oxidative stress in human melanoma cells. J Exp Ther Oncol 5: 167–181.
Newman RA, Yang P, Pawlus AD, Block KI . (2008). Cardiac glycosides as novel cancer therapeutic agents. Mol Interv 8: 36–49.
Perne A, Muellner MK, Steinrueck M, Craig-Mueller N, Mayerhofer J, Schwarzinger I et al. (2009). Cardiac glycosides induce cell death in human cells by inhibiting general protein synthesis. PLoS One 4: e8292.
Smits EL, Lee C, Hardwick N, Brooks S, Van Tendeloo VF, Orchard K et al. (2011). Clinical evaluation of cellular immunotherapy in acute myeloid leukaemia. Cancer Immunol Immunother 60: 757–769.
Stegmaier K, Corsello SM, Ross KN, Wong JS, Deangelo DJ, Golub TR . (2005). Gefitinib induces myeloid differentiation of acute myeloid leukemia. Blood 106: 2841–2848.
Tufi R, Panaretakis T, Bianchi K, Criollo A, Fazi B, Di Sano F et al. (2008). Reduction of endoplasmic reticulum Ca2+ levels favors plasma membrane surface exposure of calreticulin. Cell Death Differ 15: 274–282.
Vicencio JM, Ortiz C, Criollo A, Jones AW, Kepp O, Galluzzi L et al. (2009). The inositol 1,4,5-trisphosphate receptor regulates autophagy through its interaction with Beclin 1. Cell Death Differ 16: 1006–1017.
Vitale I, Galluzzi L, Vivet S, Nanty L, Dessen P, Senovilla L et al. (2007). Inhibition of Chk1 kills tetraploid tumor cells through a p53-dependent pathway. PLoS One 2: e1337.
Wald DN, Vermaat HM, Zang S, Lavik A, Kang Z, Peleg G et al. (2008). Identification of 6-benzylthioinosine as a myeloid leukemia differentiation-inducing compound. Cancer Res 68: 4369–4376.
Wang Z, Zheng M, Li Z, Li R, Jia L, Xiong X et al. (2009). Cardiac glycosides inhibit p53 synthesis by a mechanism relieved by Src or MAPK inhibition. Cancer Res 69: 6556–6564.
Weil M, Jacobson MD, Coles HS, Davies TJ, Gardner RL, Raff KD et al. (1996). Constitutive expression of the machinery for programmed cell death. J Cell Biol 133: 1053–1059.
Yin W, Li X, Feng S, Cheng W, Tang B, Shi YL et al. (2009). Plasma membrane depolarization and Na,K-ATPase impairment induced by mitochondrial toxins augment leukemia cell apoptosis via a novel mitochondrial amplification mechanism. Biochem Pharmacol 78: 191–202.
Zhang L, Nakaya K, Yoshida T, Kuroiwa Y . (1992). Induction by bufalin of differentiation of human leukemia cells HL60, U937, and ML1 toward macrophage/monocyte-like cells and its potent synergistic effect on the differentiation of human leukemia cells in combination with other inducers. Cancer Res 52: 4634–4641.
Acknowledgements
This work is supported by grants to GK from the Ligue Nationale contre le Cancer (Equipes labellisée), Agence Nationale pour la Recherche (ANR), European Commission (Active p53, Apo-Sys, ChemoRes, ApopTrain), Fondation pour la Recherche Médicale (FRM), Institut National du Cancer (INCa), Cancéropôle Ile-de-France, Fondation Bettencourt-Schueller and the LabEx Onco-Immunology; to VB from Agence Nationale pour la Recherche, Association pour la Recherche sur le Cancer, Belgian InterUniversity Attraction Pole and Université Paris Descartes. MT is supported by the Ligue Nationale contre le Cancer. LG is funded by Apo-Sys; KB is supported by Agence Nationale pour la Recherche.
Author contributions: MT, LS, EL, ST, DM, MS, VB, KB and OK performed the experiments; MT, LS LG and OK analyzed results and made the figures; PF, SB, LG, OK and GK designed the research and wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Tailler, M., Senovilla, L., Lainey, E. et al. Antineoplastic activity of ouabain and pyrithione zinc in acute myeloid leukemia. Oncogene 31, 3536–3546 (2012). https://doi.org/10.1038/onc.2011.521
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.521
Keywords
This article is cited by
-
SDCBP modulates tumor microenvironment, tumor progression and anti-PD1 efficacy in colorectal cancer
Cancer Gene Therapy (2024)
-
Heart failure drug proscillaridin A targets MYC overexpressing leukemia through global loss of lysine acetylation
Journal of Experimental & Clinical Cancer Research (2019)
-
Leukemic stem cell signatures identify novel therapeutics targeting acute myeloid leukemia
Blood Cancer Journal (2018)
-
Cadmium pyrithione suppresses tumor growth in vitro and in vivo through inhibition of proteasomal deubiquitinase
BioMetals (2018)
-
The role of zinc and its compounds in leukemia
JBIC Journal of Biological Inorganic Chemistry (2018)