Abstract
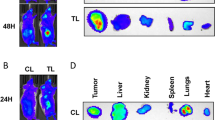
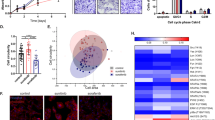
The anti-VEGF targeted antibody bevacizumab (BVZ) has been approved for treating renal cell carcinomas (RCCs). Although BVZ increases the progression-free survival of patients with metastatic RCC, the effect on overall survival is poor. To gain insight into the limited efficacy of BVZ on overall survival, we analyzed patient samples of RCC for angiogenic factors that may participate in escape from anti-VEGF therapy. Our study shows that the level of vascular endothelial growth factor (VEGF) in tumors was increased compared with normal tissue. The level of interleukin-8/CXCL8, a pro-angiogenic member of the CXCL family of cytokines, was also increased in tumors. These observations gave us a good reason to analyze the combined effects of BVZ and anti-CXCL8 antibodies on tumor growth. Surprisingly, we report that BVZ accelerates the growth of RCC in nude mice with in vivo selection of tumor cells with an increased growth capacity. Downregulation of receptor tyrosine phosphatase-κ, a phosphatase implicated in EGF receptor regulation, may partly explain this phenomenon. Modification of the vascular network and development of lymphatic vessels through VEGF-C production and compensatory production of pro-angiogenic CXCL cytokines were also observed. The apparent normalization of the vascular network prompted us to associate BVZ with the chemotherapeutic agent paclitaxel. While efficient in vitro, paclitaxel did not reverse the anti-VEGF effects in vivo. Anti-CXCL8-targeting antibodies were promising as they decreased intra-tumor VEGF production; decreased the pro-angiogenic CXCL/anti-angiogenic CXCL ratio and did not induce lymphangiogenesis. These observations hold clinical implication as they highlight putative markers implicated in escape from BVZ treatment. They also recommend proceeding with caution in the use of anti-VEGF therapy alone for treatment of RCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bertini R, Allegretti M, Bizzarri C, Moriconi A, Locati M, Zampella G et al. (2004). Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc Natl Acad Sci USA 101: 11791–11796.
Bieche I, Chavey C, Andrieu C, Busson M, Vacher S, Le Corre L et al. (2007). CXC chemokines located in the 4q21 region are upregulated in breast cancer. Endocr Relat Cancer 14: 1039–1052.
Cao Y, Wang L, Nandy D, Zhang Y, Basu A, Radisky D et al. (2008). Neuropilin-1 upholds dedifferentiation and propagation phenotypes of renal cell carcinoma cells by activating Akt and sonic hedgehog axes. Cancer Res 68: 8667–8672.
Cascone T, Herynk MH, Xu L, Du Z, Kadara H, Nilsson MB et al. (2011). Upregulated stromal EGFR and vascular remodeling in mouse xenograft models of angiogenesis inhibitor-resistant human lung adenocarcinoma. J Clin Invest 121: 1313–1328.
Cervi D, Yip TT, Bhattacharya N, Podust VN, Peterson J, Abou-Slaybi A et al. (2008). Platelet-associated PF-4 as a biomarker of early tumor growth. Blood 111: 1201–1207.
Cohen EE, Davis DW, Karrison TG, Seiwert TY, Wong SJ, Nattam S et al. (2009). Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: a phase I/II study. Lancet Oncol 10: 247–257.
Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS . (2009). Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15: 232–239.
Escudier B, Bellmunt J, Negrier S, Bajetta E, Melichar B, Bracarda S et al. (2010). Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol 28: 2144–2150.
Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C et al. (2007). Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370: 2103–2111.
Essafi-Benkhadir K, Onesto C, Stebe E, Moroni C, Pages G . (2007). Tristetraprolin inhibits Ras-dependent tumor vascularization by inducing vascular endothelial growth factor mRNA degradation. Mol Biol Cell 18: 4648–4658.
Garkavtsev I, Kozin SV, Chernova O, Xu L, Winkler F, Brown E et al. (2004). The candidate tumour suppressor protein ING4 regulates brain tumour growth and angiogenesis. Nature 428: 328–332.
Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M et al. (2010). CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest 120: 485–497.
Hainsworth JD, Spigel DR, Farley C, Thompson DS, Shipley DL, Greco FA . (2007a). Phase II trial of bevacizumab and erlotinib in carcinomas of unknown primary site: the Minnie Pearl Cancer Research Network. J Clin Oncol 25: 1747–1752.
Hainsworth JD, Spigel DR, Sosman JA, Burris 3rd HA, Farley C, Cucullu H et al. (2007b). Treatment of advanced renal cell carcinoma with the combination bevacizumab/erlotinib/imatinib: a phase I/II trial. Clin Genitourin Cancer 5: 427–432.
Harper SJ, Bates DO . (2008). VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer 8: 880–887.
Huang D, Ding Y, Zhou M, Rini BI, Petillo D, Qian CN et al. (2010). Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res 70: 1063–1071.
Jain RK . (2005). Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307: 58–62.
Joyce JA, Pollard JW . (2009). Microenvironmental regulation of metastasis. Nat Rev Cancer 9: 239–252.
Kerbel RS . (2008). Tumor angiogenesis. N Engl J Med 358: 2039–2049.
Kim JY, Song HJ, Lim HJ, Shin MG, Kim JS, Kim HJ et al. (2008). Platelet factor-4 is an indicator of blood count recovery in acute myeloid leukemia patients in complete remission. Mol Cell Proteomics 7: 431–441.
Kopetz S, Hoff PM, Morris JS, Wolff RA, Eng C, Glover KY et al. (2010). Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol 28: 453–459.
Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M et al. (2009). M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med 206: 1089–1102.
Levashova ZB, Sharma N, Timofeeva OA, Dome JS, Perantoni AO . (2007). ELR+−CXC chemokines and their receptors in early metanephric development. J Am Soc Nephrol 18: 2359–2370.
Lubner SJ, Mahoney MR, Kolesar JL, Loconte NK, Kim GP, Pitot HC et al. (2010). Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium study. J Clin Oncol 28: 3491–3497.
Merritt WM, Lin YG, Spannuth WA, Fletcher MS, Kamat AA, Han LY et al. (2008). Effect of interleukin-8 gene silencing with liposome-encapsulated small interfering RNA on ovarian cancer cell growth. J Natl Cancer Inst 100: 359–372.
Mestas J, Burdick MD, Reckamp K, Pantuck A, Figlin RA, Strieter RM . (2005). The role of CXCR2/CXCR2 ligand biological axis in renal cell carcinoma. J Immunol 175: 5351–5357.
Milanini J, Vinals F, Pouysségur J, Pagès G . (1998). p42/p44 MAP kinase module plays a key role in the transcriptional regulation of vascular endothelial growth factor gene in fibroblasts. J Biol Chem 273: 18165–18172.
Oliver G, Srinivasan RS . (2010). Endothelial cell plasticity: how to become and remain a lymphatic endothelial cell. Development 137: 363–372.
Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F et al. (2009). Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15: 220–231.
Pillai MM, Iwata M, Awaya N, Graf L, Torok-Storb B . (2006). Monocyte-derived CXCL7 peptides in the marrow microenvironment. Blood 107: 3520–3526.
Quatrale AE, Porcelli L, Silvestris N, Colucci G, Angelo A, Azzariti A . (2011). EGFR tyrosine kinases inhibitors in cancer treatment: in vitro and in vivo evidence. Front Biosci 16: 1962–1972.
Sparmann A, Bar-Sagi D . (2004). Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell 6: 447–458.
Vandercappellen J, Van Damme J, Struyf S . (2008). The role of CXC chemokines and their receptors in cancer. Cancer Lett 267: 226–244.
Xie K . (2001). Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev 12: 375–391.
Xu L, Duda DG, di Tomaso E, Ancukiewicz M, Chung DC, Lauwers GY et al. (2009). Direct evidence that bevacizumab, an anti-VEGF antibody, up-regulates SDF1alpha, CXCR4, CXCL6, and neuropilin 1 in tumors from patients with rectal cancer. Cancer Res 69: 7905–7910.
Xu Y, Tan LJ, Grachtchouk V, Voorhees JJ, Fisher GJ . (2005). Receptor-type protein-tyrosine phosphatase-kappa regulates epidermal growth factor receptor function. J Biol Chem 280: 42694–42700.
Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL et al. (2003). A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349: 427–434.
Acknowledgements
We thank Dr Marc Colombel (patients’ informed consent), Dr Jean Claude Chambard (lentivirus expressing the luciferase gene), Dr Elodie Delaplanche (biopsy management), Dr Florence Mège-Lechevallier (anatomo-pathology determination), Ms Cendrine Dubaud (animal studies) and Dr M Christiane Brahimi-Horn (careful reading of the manuscript). Financial support: Contract VEGFIL from the National Institute of Cancer (INCA), the French Association for Cancer Research (ARC, contract no. 4932) and Roche France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Grepin, R., Guyot, M., Jacquin, M. et al. Acceleration of clear cell renal cell carcinoma growth in mice following bevacizumab/Avastin treatment: the role of CXCL cytokines. Oncogene 31, 1683–1694 (2012). https://doi.org/10.1038/onc.2011.360
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.360
Keywords
This article is cited by
-
Cancer combination therapies by angiogenesis inhibitors; a comprehensive review
Cell Communication and Signaling (2022)
-
Neuropilin 1 and Neuropilin 2 gene invalidation or pharmacological inhibition reveals their relevance for the treatment of metastatic renal cell carcinoma
Journal of Experimental & Clinical Cancer Research (2021)
-
Inactivation of endothelial cell phosphoinositide 3-kinase β inhibits tumor angiogenesis and tumor growth
Oncogene (2020)
-
VEGF165b, a splice variant of VEGF-A, promotes lung tumor progression and escape from anti-angiogenic therapies through a β1 integrin/VEGFR autocrine loop
Oncogene (2019)
-
Inflammatory Indexes as Prognostic and Predictive Factors in Ovarian Cancer Treated with Chemotherapy Alone or Together with Bevacizumab. A Multicenter, Retrospective Analysis by the MITO Group (MITO 24)
Targeted Oncology (2018)