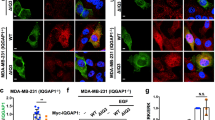
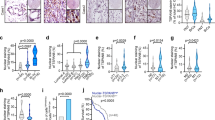
Abstract
Epidermal growth factor receptor (EGFR) family members and c-Src are co-overexpressed in many cancers. The synergistic effect of EGFR and c-Src has been shown in the tumorigenesis of breast and other cancers. Reported mechanisms of synergy include transcriptional regulation by STAT5b and the regulation of cellular ATP production by mitochondrial protein COX II. Here, we report a new mechanism of EGFR-c-Src synergy through choline kinase α (CHKA). The first enzyme of the phosphatidyl choline production pathway, CHKA, is overexpressed in many cancers, and the product of the enzyme, phosphocholine, is also increased in tumor cells. In this report, we find that CHKA forms a complex with EGFR in a c-Src-dependent manner. Endogenous CHKA and EGFR co-immunoprecipitated from a variety of breast cancer cell lines and immortalized mammary epithelial cells. CHKA interacted with the EGFR kinase domain upon c-Src co-overexpression and was phosphorylated in a c-Src-dependent manner on Y197 and Y333. Overexpression of EGFR and c-Src increased total cellular activity and protein levels of CHKA. Mutation of CHKA Y197 and Y333 reduced complex formation, EGFR-dependent activation of CHKA enzyme activity and epidermal growth factor (EGF)-dependent DNA synthesis. Furthermore, small interfering RNA-mediated knockdown of CHKA in MCF-7 and MCF-10A cells reduced EGF-dependent cell proliferation. Together, these results strongly implicate a new c-Src-dependent link between CHKA and EGFR, which contributes to the regulation of cell proliferation and tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Aoyama C, Liao H, Ishidate K . (2004). Structure and function of choline kinase isoforms in mammalian cells. Prog Lipid Res 43: 266–281.
Belsches-Jablonski AP, Biscardi JS, Peavy DR, Tice DA, Romney DA, Parsons SJ . (2001). Src family kinases and HER2 interactions in human breast cancer cell growth and survival. Oncogene 20: 1465–1475.
Biscardi JS, Belsches AP, Parsons SJ . (1998). Characterization of human epidermal growth factor receptor and c-Src interactions in human breast tumor cells. Mol Carcinog 21: 261–272.
Biscardi JS, Ishizawar RC, Silva CM, Parsons SJ . (2000). Tyrosine kinase signalling in breast cancer: epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res 2: 203–210.
Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ . (1999a). c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem 274: 8335–8343.
Biscardi JS, Tice DA, Parsons SJ . (1999b). c-Src, receptor tyrosine kinases, and human cancer. Adv Cancer Res 76: 61–119.
Boerner JL, Demory ML, Silva C, Parsons SJ . (2004). Phosphorylation of Y845 on the epidermal growth factor receptor mediates binding to the mitochondrial protein cytochrome c oxidase subunit II. Mol Cell Biol 24: 7059–7071.
Chua BT, Gallego-Ortega D, Ramirez de Molina A, Ullrich A, Lacal JC, Downward J . (2009). Regulation of Akt(ser473) phosphorylation by choline kinase in breast carcinoma cells. Mol Cancer 8: 131.
Cuadrado A, Carnero A, Dolfi F, Jimenez B, Lacal JC . (1993). Phosphorylcholine: a novel second messenger essential for mitogenic activity of growth factors. Oncogene 8: 2959–2968.
Debnath J, Muthuswamy SK, Brugge JS . (2003). Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30: 256–268.
Demory ML, Boerner JL, Davidson R, Faust W, Miyake T, Lee I et al. (2009). Epidermal growth factor receptor translocation to the mitochondria: regulation and effect. J Biol Chem 284: 36592–36604.
Dimri M, Naramura M, Duan L, Chen J, Ortega-Cava C, Chen G et al. (2007). Modeling breast cancer-associated c-Src and EGFR overexpression in human MECs: c-Src and EGFR cooperatively promote aberrant three-dimensional acinar structure and invasive behavior. Cancer Res 67: 4164–4172.
Glunde K, Ackerstaff E, Mori N, Jacobs MA, Bhujwalla ZM . (2006). Choline phospholipid metabolism in cancer: consequences for molecular pharmaceutical interventions. Mol Pharm 3: 496–506.
Grigera PR, Jeffery ED, Martin KH, Shabanowitz J, Hunt DF, Parsons JT . (2005). FAK phosphorylation sites mapped by mass spectrometry. J Cell Sci 118: 4931–4935.
Hackel PO, Gishizky M, Ullrich A . (2001). Mig-6 is a negative regulator of the epidermal growth factor receptor signal. Biol Chem 382: 1649–1662.
Hernando E, Sarmentero-Estrada J, Koppie T, Belda-Iniesta C, Ramirez de Molina V, Cejas P et al. (2009). A critical role for choline kinase-alpha in the aggressiveness of bladder carcinomas. Oncogene 28: 2425–2435.
Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR . (1989). Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77: 51–59.
Hunter T, Cooper JA . (1985). Protein-tyrosine kinases. Annu Rev Biochem 54: 897–930.
Ishidate K, Nakazawa Y . (1992). Choline/ethanolamine kinase from rat kidney. Methods Enzymol 209: 121–134.
Ishizawar R, Parsons SJ . (2004). c-Src and cooperating partners in human cancer. Cancer Cell 6: 209–214.
Ishizawar RC, Miyake T, Parsons SJ . (2007). c-Src modulates ErbB2 and ErbB3 heterocomplex formation and function. Oncogene 26: 3503–3510.
Kloth MT, Laughlin KK, Biscardi JS, Boerner JL, Parsons SJ, Silva CM . (2003). STAT5b, a mediator of synergism between c-Src and the epidermal growth factor receptor. J Biol Chem 278: 1671–1679.
Krishnamachary B, Glunde K, Wildes F, Mori N, Takagi T, Raman V et al. (2009). Noninvasive detection of lentiviral-mediated choline kinase targeting in a human breast cancer xenograft. Cancer Res 69: 3464–3471.
Liao H, Aoyama C, Ishidate K, Teraoka H . (2006). Deletion and alanine mutation analyses for the formation of active homo- or hetero-dimer complexes of mouse choline kinase-alpha and -beta. Biochim Biophys Acta 1761: 111–120.
Maa MC, Leu TH, McCarley DJ, Schatzman RC, Parsons SJ . (1995). Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: implications for the etiology of multiple human cancers. Proc Natl Acad Sci U S A 92: 6981–6985.
Malito E, Sekulic N, Too WC, Konrad M, Lavie A . (2006). Elucidation of human choline kinase crystal structures in complex with the products ADP or phosphocholine. J Mol Biol 364: 136–151.
Mao W, Irby R, Coppola D, Fu L, Wloch M, Turner J et al. (1997). Activation of c-Src by receptor tyrosine kinases in human colon cancer cells with high metastatic potential. Oncogene 15: 3083–3090.
Morse DL, Carroll D, Day S, Gray H, Sadarangani P, Murthi S et al. (2009). Characterization of breast cancers and therapy response by MRS and quantitative gene expression profiling in the choline pathway. NMR Biomed 22: 114–127.
Peisach D, Gee P, Kent C, Xu Z . (2003). The crystal structure of choline kinase reveals a eukaryotic protein kinase fold. Structure 11: 703–713.
Qiu C, Tarrant MK, Boronina T, Longo PA, Kavran JM, Cole RN et al. (2009). In vitro enzymatic characterization of near full length EGFR in activated and inhibited states. Biochemistry 48: 6624–6632.
Ramirez de Molina A, Banez-Coronel M, Gutierrez R, Rodriguez-Gonzalez A, Olmeda D, Megias D et al. (2004). Choline kinase activation is a critical requirement for the proliferation of primary human mammary epithelial cells and breast tumor progression. Cancer Res 64: 6732–6739.
Ramirez de Molina A, Gutierrez R, Ramos MA, Silva JM, Silva J, Bonilla F et al. (2002a). Increased choline kinase activity in human breast carcinomas: clinical evidence for a potential novel antitumor strategy. Oncogene 21: 4317–4322.
Ramirez de Molina A, Penalva V, Lucas L, Lacal JC . (2002b). Regulation of choline kinase activity by Ras proteins involves Ral-GDS and PI3K. Oncogene 21: 937–946.
Ramirez de Molina A, Rodriguez-Gonzalez A, Gutierrez R, Martinez-Pineiro L, Sanchez J, Bonilla F et al. (2002c). Overexpression of choline kinase is a frequent feature in human tumor-derived cell lines and in lung, prostate, and colorectal human cancers. Biochem Biophys Res Commun 296: 580–583.
Ramirez de Molina A, Sarmentero-Estrada J, Belda-Iniesta C, Taron M, Ramirez de Molina V, Cejas P et al. (2007). Expression of choline kinase alpha to predict outcome in patients with early-stage non-small-cell lung cancer: a retrospective study. Lancet Oncol 8: 889–897.
Stover DR, Becker M, Liebetanz J, Lydon NB . (1995). Src phosphorylation of the epidermal growth factor receptor at novel sites mediates receptor interaction with Src and P85 alpha. J Biol Chem 270: 15591–15597.
Tice DA, Biscardi JS, Nickles AL, Parsons SJ . (1999). Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci U S A 96: 1415–1420.
Uchida T . (1996). Stimulation of phospholipid synthesis in HeLa cells by epidermal growth factor and insulin: activation of choline kinase and glycerophosphate acyltransferase. Biochim Biophys Acta 1304: 89–104.
Wright JD, Reuter CW, Weber MJ . (1996). Identification of sites on epidermal growth factor receptors which are phosphorylated by pp60src in vitro. Biochim Biophys Acta 1312: 85–93.
Wu G, Vance DE . (2010). Choline kinase and its function. Biochem Cell Biol 88: 559–564.
Yalcin A, Clem B, Makoni S, Clem A, Nelson K, Thornburg J et al. (2010). Selective inhibition of choline kinase simultaneously attenuates MAPK and PI3K/AKT signaling. Oncogene 29: 139–149.
Yang S, Park K, Turkson J, Arteaga CL . (2008). Ligand-independent phosphorylation of Y869 (Y845) links mutant EGFR signaling to stat-mediated gene expression. Exp Cell Res 314: 413–419.
Acknowledgements
We thank Dr Nicholas E Sherman in the WM Keck Biomedical Mass Spectrometry Laboratory for analysis of CHKA2 phosphorylation sites, and Drs Jill Slack-Davis and John DaSilva for their critical comments. We also thank members of Sarah J Parsons’ laboratory and the Women's Oncology Group in the University of Virginia Cancer Center for helpful discussions. This work was supported by NIH-NCI grant R01CA123037(SJP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Miyake, T., Parsons, S. Functional interactions between Choline kinase α, epidermal growth factor receptor and c-Src in breast cancer cell proliferation. Oncogene 31, 1431–1441 (2012). https://doi.org/10.1038/onc.2011.332
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.332
Keywords
This article is cited by
-
Identification and validation of novel and more effective choline kinase inhibitors against Streptococcus pneumoniae
Scientific Reports (2020)
-
Molecular basis for the interaction between human choline kinase alpha and the SH3 domain of the c-Src tyrosine kinase
Scientific Reports (2019)
-
Choline kinase inhibition and docking studies of a series of 6-(benzylthio)-9H-purin-9-yl-pyridinium derivatives
Medicinal Chemistry Research (2017)
-
Identification of putative target genes for amplification within 11q13.2 and 3q27.1 in esophageal squamous cell carcinoma
Clinical and Translational Oncology (2014)
-
A non-catalytic role of choline kinase alpha is important in promoting cancer cell survival
Oncogenesis (2013)