Abstract
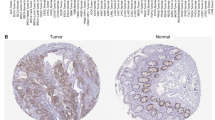
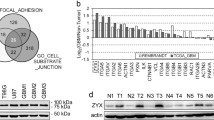
Spindle cell sarcomas consist of tumors with different biological features, of which distant metastasis is the most ominous sign for a poor prognosis. However, metastasis is difficult to predict on the basis of current histopathological analyses. We have identified actin filament-associated protein 1-like 1 (AFAP1L1) as a candidate for a metastasis-predicting marker from the gene expression profiles of 65 spindle cell sarcomas. A multivariate analysis determined that AFAP1L1 was an independent factor for predicting the occurrence of distant metastasis (P=0.0001), which was further confirmed in another set of 41 tumors by a quantitative mRNA expression analysis. Immunohistochemical staining using paraffin-embedded tumor tissues revealed that the metastasis-free rate was significantly better in tumors negative for AFAP1L1 (P=0.0093 by log-rank test). Knocking down the AFAP1L1 gene in sarcoma cells resulted in inhibition of the cell invasion, and forced expression of AFAP1L1 in immortalized human mesenchymal stem cells induced anchorage-independent growth and increased cell invasiveness with high activity levels of matrix metallopeptidase. Furthermore, tumor growth in vivo was accelerated in AFAP1L1-transduced sarcoma cell lines. These results suggest that AFAP1L1 has a role in the progression of spindle cell sarcomas and is a prognostic biomarker.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Aoyama T, Okamoto T, Nagayama S, Nishijo K, Ishibe T, Yasura K et al. (2004). Methylation in the core-promoter region of the chondromodulin-I gene determines the cell-specific expression by regulating the binding of transcriptional activator, Sp3. J Biol Chem 279: 28789–28797.
Baisden JM, Gatesman AS, Cherezova L, Jiang BH, Flynn DC . (2001b). The intrinsic ability of AFAP-110 to alter actin filament integrity is linked with its ability to also activate cellular tyrosine kinases. Oncogene 20: 6607–6616.
Baisden JM, Qian Y, Zot HM, Flynn DC . (2001a). The actin filament-associated protein AFAP-110 is an adaptor protein that modulates changes in actin filament integrity. Oncogene 20: 6435–6447.
Barretina J, Taylor BS, Banerji S, Ramos AH, Lagos-Quintana M, DeCarolis PL et al. (2010). Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet 42: 715–721.
Chibon F, Lagarde P, Salas S, Pérot G, Brouste V, Tirode F et al. (2010). Validated prediction of clinical outcome in sarcomas and multiple types of cancer on the basis of a gene expression signature related to genome complexity. Nat Med 16: 781–787.
Clark J, Rocques PJ, Crew AJ, Gill S, Shipley J, Chan AM et al. (1994). Identification of novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nat Genet 7: 502–508.
Dorfleutner A, Stehlik C, Zhang J, Gallick GE, Flynn DC . (2007). AFAP-110 is required for actin stress fiber formation and cell adhesion in MDA-MB-231 breast cancer cells. J Cell Physiol 213: 740–749.
Egeblad M, Werb Z . (2002). New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2: 161–174.
Francis P, Namlos HM, Muller C, Eden P, Fernebro J, Berner JM et al. (2007). Diagnostic and prognostic gene expression signatures in 177 soft tissue sarcomas: hypoxia-induced transcription profile signifies metastatic potential. BMC Genomics 8: 73.
Fletcher CD, Gustafson P, Rydholm A, Willen H, Akerman M . (2001). Clinicopathologic re-evaluation of 100 malignant fibrous histiocytomas: prognostic relevance of subclassification. J Clin Oncol 19: 3045–3050.
Fletcher CDM, Unni KK, Mertens F . (2002). Pathology and Genetics of Tumours of Soft Tissue and Bones. IARC Press: Lyon.
Flynn DC, Leu TH, Reynolds AB, Parsons JT . (1993). Identification and sequence analysis of cDNAs encoding a 110-kilodalton actin filament-associated pp60src substrate. Mol Cell Biol 13: 7892–7900.
Gatesman A, Walker VG, Baisden JM, Weed SA, Flynn DC . (2004). Protein kinase Calpha activates c-Src and induces podosome formation via AFAP-110. Mol Cell Biol 24: 7578–7597.
Guappone AC, Flynn DC . (1997). The integrity of the SH3 binding motif of AFAP-110 is required to facilitate tyrosine phosphorylation by, and stable complex formation with, Src. Mol Cell Biochem 175: 243–252.
Helman LJ, Meltzer P . (2003). Mechanisms of sarcoma development. Nat Rev Cancer 3: 685–694.
Ishibe T, Nakayama T, Aoyama T, Nakamura T, Toguchida J . (2008). Neuronal differentiation of synovial sarcoma and its therapeutic application. Clin Orthop Relat Res 466: 2147–2155.
Ishibe T, Nakayama T, Okamoto T, Aoyama T, Nishijo K, Shibata KR et al. (2005). Disruption of fibroblast growth factor signal pathway inhibits the growth of synovial sarcomas: potential application of signal inhibitors to molecular target therapy. Clin Cancer Res 11: 2702–2712.
Kanner SB, Reynolds AB, Vines RR, Parsons JT . (1990). Monoclonal antibodies to individual tyrosine-phosphorylated protein substrated of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci USA 87: 3328–3332.
Kohno Y, Okamoto T, Ishibe T, Nagayama S, Shima Y, Nishijo K et al. (2006). Expression of claudin7 is tightly associated with epithelial structures in synovial sarcomas and regulated by an Ets family transcription factor, ELF3. J Biol Chem 281: 38941–38950.
Lee YF, John M, Falconer A, Edwards S, Clark J, Flohr P et al. (2004). A gene expression signature associated with metastatic outcome in human leiomyosarcomas. Cancer Res 64: 7201–7204.
Lodyga M, De Falco V, Bai XH, Kapus A, Melillo RM, Santoro M et al. (2009). XB130, a tissue-specific adaptor protein that couples the RET/PTC oncogenic kinase to PI 3-kinase pathway. Oncogene 28: 937–949.
Matushansky I, Hernando E, Socci ND, Mills JE, Matos TA, Edgar MA et al. (2007). Derivation of sarcomas from mesenchymal stem cells via inactivation of the Wnt pathway. J Clin Invest 117: 3248–3257.
Nagayama S, Fukukawa C, Katagiri T, Okamoto T, Aoyama T, Oyaizu N et al. (2005). Therapeutic potential of antibodies against FZD 10, a cell-surface protein, for synovial sarcomas. Oncogene 24: 6201–6212.
Nagayama S, Katagiri T, Tsunoda T, Hosaka T, Nakashima Y, Araki N et al. (2002). Genome-wide analysis of gene expression in synovial sarcomas using a cDNA microarray. Cancer Res 62: 5859–5866.
Nakayama R, Nemoto T, Takahashi H, Ohta T, Kawai A, Seki K et al. (2007). Gene expression analysis of soft tissue sarcomas: characterization and reclassification of malignant fibrous histiocytoma. Mod Pathol 20: 749–759.
Nielsen TO, West RB, Linn SC, Alter O, Knowling MA, O'Connell JX et al. (2002). Molecular characterization of soft tissue tumours: a gene expression study. Lancet 359: 1301–1307.
Ottaiano A, De Chiara A, Fazioli F, Talamanca AA, Mori S, Botti G et al. (2005). Biological prognostic factors in adult soft tissue sarcomas. Anticancer Res 25: 4519–4526.
Overall CM, Kleifeld O . (2006). Tumour microenvironment—opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer 6: 227–239.
Qian Y, Baisden JM, Westin EH, Guappone AC, Koay TC, Flynn DC . (1998). Src can regulate carboxy terminal interactions with AFAP-110, which influence self-association, cell localization and actin filament integrity. Oncogene 16: 2185–2195.
Qian Y, Baisden JM, Zot HG, Van Winkle WB, Flynn DC . (2000). The carboxy terminus of AFAP-110 modulates direct interactions with actin filaments and regulates its ability to alter actin filament integrity and induce lamellipodia formation. Exp Cell Res 255: 102–113.
Qian Y, Gatesman AS, Baisden JM, Zot HG, Cherezova L, Qazi I et al. (2004). Analysis of the role of the leucine zipper motif in regulating the ability of AFAP-110 to alter actin filament integrity. J Cell Biochem 91: 602–620.
Shibata KR, Aoyama T, Shima Y, Fukiage K, Otsuka S, Furu M et al. (2007). Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem Cells 25: 2371–2382.
Shima Y, Okamoto T, Aoyama T, Yasura K, Ishibe T, Nishijo K et al. (2007). In vitro transformation of mesenchymal stem cells by oncogenic H-rasVal12. Biochem Biophys Res Commun 353: 60–66.
St-Pierre Y, Couillard J, Van Themsche C . (2004). Regulation of MMP-9 gene expression for the development of novel molecular targets against cancer and inflammatory diseases. Expert Opin Ther Targets 8: 473–489.
Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S et al. (2006). Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell 9: 287–300.
Toguchida J, Nakayama T . (2009). Molecular genetics of sarcomas: applications to diagnoses and therapy. Cancer Sci 100: 1573–1580.
Toguchida J, Yamaguchi T, Ritchie B, Beauchamp RL, Dayton SH, Herrera GE et al. (1992). Mutation spectrum of the p53 gene in bone and soft tissue sarcomas. Cancer Res 52: 6194–6199.
Wadayama B, Toguchida J, Shimizu T, Ishizaki K, Sasaki MS, Kotoura Y et al. (1994). Mutation spectrum of the retinoblastoma gene in osteosarcomas. Cancer Res 54: 3042–3048.
Xu J, Bai XH, Lodyga M, Han B, Xiao H, Keshavjee S et al. (2007). XB130, a novel adaptor protein for signal transduction. J Biol Chem 282: 16401–16412.
Zhang J, Park SI, Artime MC, Summy JM, Shah AN, Bomser JA et al. (2007). AFAP-110 is overexpressed in prostate cancer and contributes to tumorigenic growth by regulating focal contacts. J Clin Invest 117: 2962–2973.
Acknowledgements
We thank Drs H Sonobe, A Kawai, S Tanaka, O Larsson, A Ogose, and H Kanda for providing cell lines, and T Tsunoda and S Miyano for data analyses. This work was supported by Grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Furu, M., Kajita, Y., Nagayama, S. et al. Identification of AFAP1L1 as a prognostic marker for spindle cell sarcomas. Oncogene 30, 4015–4025 (2011). https://doi.org/10.1038/onc.2011.108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.108
Keywords
This article is cited by
-
AFAP1L1 promotes gastric cancer progression by interacting with VAV2 to facilitate CDC42-mediated activation of ITGA5 signaling pathway
Journal of Translational Medicine (2023)
-
Regulation of sarcoma cell migration, invasion and invadopodia formation by AFAP1L1 through a phosphotyrosine-dependent pathway
Oncogene (2016)
-
LASS2 enhances chemosensitivity of breast cancer by counteracting acidic tumor microenvironment through inhibiting activity of V-ATPase proton pump
Oncogene (2013)
-
Sarcoma spreads primarily through the vascular system: are there biomarkers associated with vascular spread?
Clinical & Experimental Metastasis (2012)