Abstract
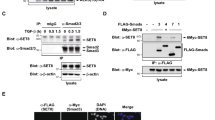
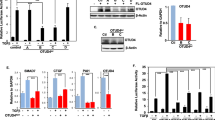
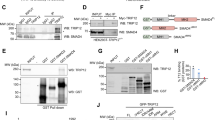
Ubiquitin-dependent mechanisms have emerged as essential regulatory elements controlling cellular levels of Smads and TGFβ-dependent biological outputs such as epithelial–mesenchymal transition (EMT). In this study, we identify a HECT E3 ubiquitin ligase known as WWP2 (Full-length WWP2-FL), together with two WWP2 isoforms (N-terminal, WWP2-N; C-terminal WWP2-C), as novel Smad-binding partners. We show that WWP2-FL interacts exclusively with Smad2, Smad3 and Smad7 in the TGFβ pathway. Interestingly, the WWP2-N isoform interacts with Smad2 and Smad3, whereas WWP2-C interacts only with Smad7. In addition, WWP2-FL and WWP2-C have a preference for Smad7 based on protein turnover and ubiquitination studies. Unexpectedly, we also find that WWP2-N, which lacks the HECT ubiquitin ligase domain, can also interact with WWP2-FL in a TGFβ-regulated manner and activate endogenous WWP2 ubiquitin ligase activity causing degradation of unstimulated Smad2 and Smad3. Consistent with our protein interaction data, overexpression and knockdown approaches reveal that WWP2 isoforms differentially modulate TGFβ-dependent transcription and EMT. Finally, we show that selective disruption of WWP2 interactions with inhibitory Smad7 can stabilise Smad7 protein levels and prevent TGFβ-induced EMT. Collectively, our data suggest that WWP2-N can stimulate WWP2-FL leading to increased activity against unstimulated Smad2 and Smad3, and that Smad7 is a preferred substrate for WWP2-FL and WWP2-C following prolonged TGFβ stimulation. Significantly, this is the first report of an interdependent biological role for distinct HECT E3 ubiquitin ligase isoforms, and highlights an entirely novel regulatory paradigm that selectively limits the level of inhibitory and activating Smads.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Atsumi T, Miwa Y, Kimata K, Ikawa Y . (1990). A chondrogenic cell line derived from a differentiating culture of AT805 teratocarcinoma cells. Cell Differ Dev 30: 109–116.
Bernassola F, Karin M, Ciechanover A, Melino G . (2008). The HECT family of E3 ubiquitin ligases: multiple players in cancer development. Cancer Cell 14: 10–21.
Bonni S, Wang HR, Causing CG, Kavsak P, Stroschein SL, Luo K et al. (2001). TGF-beta induces assembly of a Smad2-Smurf2 ubiquitin ligase complex that targets SnoN for degradation. Nat Cell Biol 3: 587–595.
Danckwardt S, Hentze MW, Kulozik AE . (2008). 3′ end mRNA processing: molecular mechanisms and implications for health and disease. EMBO Journal 27: 482–498.
Ellenrieder V, Hendler SF, Boeck W, Seufferlein WT, Menke A, Ruhland C et al. (2001). Transforming growth factor beta1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation. Cancer Res 61: 4222–4228.
Flasza M, Gorman P, Roylance R, Canfield AE, Baron M . (2002). Alternative splicing determines the domain structure of WWP1, a Nedd4 family protein. Biochem Biophys Res Commun 290: 431–437.
Foot NJ, Dalton HE, Shearwin-Whyatt LM, Dorstyn L, Tan SS, Yang B et al. (2008). Regulation of the divalent metal ion transporter DMT1 and iron homeostasis by a ubiquitin-dependent mechanism involving Ndfips and WWP2. Blood 112: 4268–4275.
Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH et al. (2000). Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol Cell 6: 1365–1375.
Hata A, Lo RS, Wotton D, Lagna G, Massagué J . (1997). Mutations increasing autoinhibition inactivate tumour suppressors Smad2 and Smad4. Nature 388: 82–87.
Heldin CH, Landström M, Moustakas A . (2009). Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol 21: 166–176.
Hong S, Lee C, Kim SJ . (2007). Smad7 sensitizes tumor necrosis factor induced apoptosis through the inhibition of anti-apoptotic gene expression by suppressing activation of the nuclear factor-kappaB pathway. Cancer Res 67: 9577–9583.
Javelaud D, Mohammad KS, McKenna CR, Fournier P, Luciani F, Niewolna M et al. (2007). Stable expression of Smad7 in human melanoma cells impairs bone metastasis. Cancer Res 67: 2317–2324.
Levy L, Hill CS . (2005). Smad4 dependency defines two classes of transforming growth factor-β target genes and distinguishes TGF-β-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol Cell Biol 25: 8108–8125.
Li W, Bengtson MH, Ulbrich A, Matsuda A, Reddy VA, Orth A et al. (2008). Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle’ dynamics and signaling. PLoS ONE 3: e1487.
Li H, Zhang Z, Wang B, Zhang J, Zhao Y, Jin Y . (2007). Wwp2-mediated ubiquitination of the RNA polymerase II large subunit in mouse embryonic pluripotent stem cells. Mol Cell Biol 27: 5296–5305.
Lönn P, Morén A, Raja E, Dahl M, Moustakas A . (2009). Regulating the stability of TGFbeta receptors and Smads. Cell Res 19: 21–35.
Lu PJ, Zhou XZ, Shen M, Lu KP . (1999). Function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science 283: 1325–1328.
Lutz CS . (2008). Alternative polyadenylation: A twist on mRNA 3′ end formation. ACS Chem Biol 3: 609–617.
McDonald FJ, Western AH, McNeil JD, Thomas BC, Olson DR, Snyder PM . (2002). Ubiquitin-protein ligase WWP2 binds to and downregulates the epithelial Na(+) channel. Am J Physiol Renal Physiol 283: F431–F436.
Mund T, Pelham HR . (2009). Control of the activity of WW-HECT domain E3 ubiquitin ligases by NDFIP proteins. EMBO Rep 10: 501–507.
Murray-Zmijewski F, Lane DP, Bourdon JC . (2006). p53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ 13: 962–972.
Nilsen TW, Graveley BR . (2010). Expansion of the eukaryotic proteome by alternative splicing. Nature 463: 457–463.
Pirozzi G, McConnell SJ, Uveges AJ, Carter JM, Sparks AB, Kay BK et al. (1997). Identification of novel human WW domain-containing proteins by cloning of ligand targets. J Biol Chem 272: 14611–14616.
Raikwar NS, Thomas CP . (2008). Nedd4-2 isoforms ubiquitinate individual epithelial sodium channel subunits and reduce surface expression and function of the epithelial sodium channel. Am J Physiol Renal Physiol 294: 1157–1165.
Tazi J, Bakkour N, Stamm S . (2009). Alternative splicing and disease. Biochimica et Biophysica Acta 1792: 14–26.
Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A . (2005). TGF-beta and the Smad signalling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell 16: 1987–2002.
Wicks S, Haros K, Maillard M, Song L, Cohen RE, ten Dijke P et al. (2005). The deubiquitinating enzyme UCH37 interacts with Smads and regulates TGFβ signalling. Oncogene 24: 8080–8084.
Wiesner S, Ogunjimi AA, Wang HR, Rotin D, Sicheri F, Wrana JL et al. (2007). Autoinhibition of the HECT-type ubiquitin ligase Smurf2 through its C2 domain. Cell 130: 651–662.
Xu H, Wang W, Li C, Yu H, Yang A, Wang B et al. (2009a). WWP2 promotes degradation of transcription factor OCT4 in human embryonic stem cells. Cell Res 19: 561–573.
Xu HM, Liao B, Zhang QJ, Wang BB, Li H, Zhong XM et al. (2004). Wwp2, an E3 ubiquitin ligase that targets transcription factor Oct-4 for ubiquitination. J Biol Chem 279: 23495–23503.
Xu J, Lamouille S, Derynck R . (2009b). TGF-beta-induced epithelial to mesenchymal transition. Cell Res 19: 156–172.
Zavadil J, Böttinger EP . (2005). TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 24: 5764–5774.
Zhang S, Ekman M, Thakur N, Bu S, Davoodpour P, Grimsby S et al. (2006). TGFbeta-induced activation of ATM and p53 mediates apoptosis in a Smad7-dependent manner. Cell Cycle 5: 2787–2795.
Zhu H, Kavsak P, Abdollah S, Wrana JL, Thomsen GH . (1999). A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature 400: 687–693.
Acknowledgements
We thank Fiona McDonald, Caroline Hill, and Hans Clevers for generously providing cells/plasmids, and Ian Clark and Tracey Swingler for generously providing ATDC5 cDNA samples. This work was supported by the Association for International Research (AICR), with additional funding provided by the BigC Charity, the British Skin Foundation and the Dunhill Medical Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website ()
Supplementary information
Rights and permissions
About this article
Cite this article
Soond, S., Chantry, A. Selective targeting of activating and inhibitory Smads by distinct WWP2 ubiquitin ligase isoforms differentially modulates TGFβ signalling and EMT. Oncogene 30, 2451–2462 (2011). https://doi.org/10.1038/onc.2010.617
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.617
Keywords
This article is cited by
-
WWP2 drives the progression of gastric cancer by facilitating the ubiquitination and degradation of LATS1 protein
Cell Communication and Signaling (2023)
-
The role of NEDD4 related HECT-type E3 ubiquitin ligases in defective autophagy in cancer cells: molecular mechanisms and therapeutic perspectives
Molecular Medicine (2023)
-
Primate-specific isoform of Nedd4-1 regulates substrate binding via Ser/Thr phosphorylation and 14-3-3 binding
Scientific Reports (2023)
-
WWP2 Is One Promising Novel Oncogene
Pathology & Oncology Research (2019)
-
WWP2 regulates pathological cardiac fibrosis by modulating SMAD2 signaling
Nature Communications (2019)