Abstract
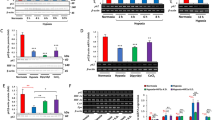
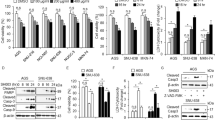
Sequestosome 1 (SQSTM1/p62) is a multifunctional protein involved in signal transduction, protein degradation and cell transformation. Hypoxia is a common feature of solid tumours that promotes cancer progression. Here, we report that p62 is downregulated in hypoxia in carcinoma cells and that the expression is rapidly restored in response to reoxygenation. The hypoxic p62 downregulation did not occur at the mRNA level and was independent of the hypoxic signal mediators hypoxia-inducible factor (HIF) and von Hippel-Lindau tumour suppressor protein as well as the activity of HIF-prolyl hydroxylases and was not mediated by proteosomal destruction. Autophagy was activated in hypoxia and was required for p62 degradation. The hypoxic degradation of p62 was blocked by autophagy inhibitors as well as by the attenuation of Atg8/LC3 expression. Downregulation of p62 was required for hypoxic extracellular regulated kinase (ERK)-1/2 phosphorylation. Attenuation of p62 in normoxia activated and forced expression of p62 in hypoxia blocked the activation of ERK-1/2. The results demonstrate that hypoxic activation of autophagy induces clearance of p62 protein and implies a role for p62 in the regulation of hypoxic cancer cell survival responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN et al. (2006). Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol 169: 566–583.
Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J et al. (2003). HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J 22: 4082–4090.
Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A et al. (2005). p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171: 603–614.
Bruick RK, McKnight SL . (2001). A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294: 1337–1340.
Duran A, Linares JF, Galvez AS, Wikenheiser K, Flores JM, Diaz-Meco MT et al. (2008). The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell 13: 343–354.
Duran A, Serrano M, Leitges M, Flores JM, Picard S, Brown JP et al. (2004). The atypical PKC-interacting protein p62 is an important mediator of RANK-activated osteoclastogenesis. Dev Cell 6: 303–309.
Ekstrom P, Kanje M . (1984). Inhibition of fast axonal transport by erythro-9-[3-(2-hydroxynonyl)]adenine. J Neurochem 43: 1342–1345.
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR et al. (2001). C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54.
Geetha T, Jiang J, Wooten MW . (2005). Lysine 63 polyubiquitination of the nerve growth factor receptor TrkA directs internalization and signaling. Mol Cell 20: 301–312.
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M et al. (2001). HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468.
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ et al. (2001). Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472.
Jokilehto T, Rantanen K, Luukkaa M, Heikkinen P, Grenman R, Minn H et al. (2006). Overexpression and nuclear translocation of hypoxia-inducible factor prolyl hydroxylase PHD2 in head and neck squamous cell carcinoma is associated with tumor aggressiveness. Clin Cancer Res 12: 1080–1087.
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T et al. (2000). LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19: 5720–5728.
Kaelin WG . (2005). Proline hydroxylation and gene expression. Annu Rev Biochem 74: 115–128.
Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T et al. (2007). Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131: 1149–1163.
Kondo Y, Kanzawa T, Sawaya R, Kondo S . (2005). The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 5: 726–734.
Koumenis C, Wouters BG . (2006). ‘Translating’ tumor hypoxia: unfolded protein response (UPR)-dependent and UPR-independent pathways. Mol Cancer Res 4: 423–436.
Kuusisto E, Salminen A, Alafuzoff I . (2001a). Ubiquitin-binding protein p62 is present in neuronal and glial inclusions in human tauopathies and synucleinopathies. NeuroReport 12: 2085–2090.
Kuusisto E, Suuronen T, Salminen A . (2001b). Ubiquitin-binding protein p62 expression is induced during apoptosis and proteasomal inhibition in neuronal cells. Biochem Biophys Res Commun 280: 223–228.
Kwon DS, Kwon CH, Kim JH, Woo JS, Jung JS, Kim YK . (2006). Signal transduction of MEK/ERK and PI3K/Akt activation by hypoxia/reoxygenation in renal epithelial cells. Eur J Cell Biol 85: 1189–1199.
Lamark T, Perander M, Outzen H, Kristiansen K, Overvatn A, Michaelsen E et al. (2003). Interaction codes within the family of mammalian Phox and Bem1p domain-containing proteins. J Biol Chem 278: 34568–34581.
Levine B, Kroemer G . (2008). Autophagy in the pathogenesis of disease. Cell 132: 27–42.
Lim JH, Park JW, Kim MS, Park SK, Johnson RS, Chun YS . (2006). Bafilomycin induces the p21-mediated growth inhibition of cancer cells under hypoxic conditions by expressing hypoxia-inducible factor-1alpha. Mol Pharmacol 70: 1856–1865.
Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC . (2006). Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell 21: 521–531.
Martin P, Diaz-Meco MT, Moscat J . (2006). The signaling adapter p62 is an important mediator of T helper 2 cell function and allergic airway inflammation. EMBO J 25: 3524–3533.
Masson N, Appelhoff RJ, Tuckerman JR, Tian YM, Demol H, Puype M et al. (2004). The HIF prolyl hydroxylase PHD3 is a potential substrate of the TRiC chaperonin. FEBS Lett 570: 166–170.
Minet E, Arnould T, Michel G, Roland I, Mottet D, Raes M et al. (2000). ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett 468: 53–58.
Mizushima N, Levine B, Cuervo AM, Klionsky DJ . (2008). Autophagy Figurehts disease through cellular self-digestion. Nature 451: 1069–1075.
Nakaso K, Yoshimoto Y, Nakano T, Takeshima T, Fukuhara Y, Yasui K et al. (2004). Transcriptional activation of p62/A170/ZIP during the formation of the aggregates: possible mechanisms and the role in Lewy body formation in Parkinson's disease. Brain Res 1012: 42–51.
Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T et al. (2005). Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science 307: 593–596.
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H et al. (2007). p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282: 24131–24145.
Pugh CW, Ratcliffe PJ . (2003). Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 9: 677–684.
Rodriguez A, Duran A, Selloum M, Champy MF, Diez-Guerra FJ, Flores JM et al. (2006). Mature-onset obesity and insulin resistance in mice deficient in the signaling adapter p62. Cell Metab 3: 211–222.
Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z . (2007). Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J 26: 1749–1760.
Schofield CJ, Ratcliffe PJ . (2004). Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 5: 343–354.
Seglen PO, Gordon PB . (1982). 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA 79: 1889–1892.
Seibenhener ML, Babu JR, Geetha T, Wong HC, Krishna NR, Wooten MW . (2004). Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol Cell Biol 24: 8055–8068.
Semenza GL . (2003). Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3: 721–732.
Thompson HG, Harris JW, Wold BJ, Lin F, Brody JP . (2003). p62 overexpression in breast tumors and regulation by prostate-derived Ets factor in breast cancer cells. Oncogene 22: 2322–2333.
Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC . (2006). Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res 99: 275–282.
Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, Macleod KF . (2007). BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol 27: 6229–6242.
Wooten MW, Hu X, Babu JR, Seibenhener ML, Geetha T, Paine MG et al. (2006). Signaling, polyubiquitination, trafficking, and inclusions: sequestosome 1/p62's role in neurodegenerative disease. J Biomed Biotechnol 2006: 62079.
Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y . (1998). Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct 23: 33–42.
Zatloukal K, Stumptner C, Fuchsbichler A, Heid H, Schnoelzer M, Kenner L et al. (2002). p62 Is a common component of cytoplasmic inclusions in protein aggregation diseases. Am J Pathol 160: 255–263.
Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB et al. (2008). Mitochondrial autophagy is a HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem 283: 10892–10903.
Acknowledgements
Taina Kalevo-Mattila is acknowledged for expert technical assistance. The study was supported by The Academy of Finland (grants 200779, 210282 and 8109024), Emil Aaltonen Foundation and Sigrid Juselius Foundation to PMJ, and by grants from the Norwegian Research Council (FUGE) and Norwegian Cancer Society to TJ. PMJ is a research fellow of the Finnish Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Rights and permissions
About this article
Cite this article
Pursiheimo, JP., Rantanen, K., Heikkinen, P. et al. Hypoxia-activated autophagy accelerates degradation of SQSTM1/p62. Oncogene 28, 334–344 (2009). https://doi.org/10.1038/onc.2008.392
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.392
Keywords
This article is cited by
-
Galectins and galectin-mediated autophagy regulation: new insights into targeted cancer therapy
Biomarker Research (2023)
-
Ubiquitination of MAP1LC3B by pVHL is associated with autophagy and cell death in renal cell carcinoma
Cell Death & Disease (2019)
-
Both p62/SQSTM1-HDAC6-dependent autophagy and the aggresome pathway mediate CDK1 degradation in human breast cancer
Scientific Reports (2017)
-
Mycobacterium tuberculosis PE_PGRS41 Enhances the Intracellular Survival of M. smegmatis within Macrophages Via Blocking Innate Immunity and Inhibition of Host Defense
Scientific Reports (2017)
-
BIRC3 is a biomarker of mesenchymal habitat of glioblastoma, and a mediator of survival adaptation in hypoxia-driven glioblastoma habitats
Scientific Reports (2017)