Abstract
Tethering telomeres to the inner nuclear membrane (INM) allows homologous chromosome pairing during meiosis. The meiosis-specific protein TERB1 binds the telomeric protein TRF1 to establish telomere–INM connectivity and is essential for mouse fertility. Here we solve the structure of the human TRF1–TERB1 interface to reveal the structural basis for telomere–INM linkage. Disruption of this interface abrogates binding and compromises telomere–INM attachment in mice. An embedded CDK-phosphorylation site within the TRF1-binding region of TERB1 provides a mechanism for cap exchange, a late-pachytene phenomenon involving the dissociation of the TRF1–TERB1 complex. Indeed, further strengthening this interaction interferes with cap exchange. Finally, our biochemical analysis implicates distinct complexes for telomere–INM tethering and chromosome-end protection during meiosis. Our studies unravel the structure, stoichiometry, and physiological implications underlying telomere–INM tethering, thereby providing unprecedented insights into the unique function of telomeres in meiosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Blackburn, E.H. Switching and signaling at the telomere. Cell 106, 661–673 (2001).
Makarov, V.L., Hirose, Y. & Langmore, J.P. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell 88, 657–666 (1997).
Palm, W. & de Lange, T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 42, 301–334 (2008).
Bilaud, T. et al. Telomeric localization of TRF2, a novel human telobox protein. Nat. Genet. 17, 236–239 (1997).
Broccoli, D., Smogorzewska, A., Chong, L. & de Lange, T. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat. Genet. 17, 231–235 1997).
Nishikawa, T. et al. Solution structure of a telomeric DNA complex of human TRF1. Structure 9, 1237–1251 (2001).
Nandakumar, J. & Cech, T.R. Finding the end: recruitment of telomerase to telomeres. Nat. Rev. Mol. Cell Biol. 14, 69–82 (2013).
Watanabe, Y. Geometry and force behind kinetochore orientation: lessons from meiosis. Nat. Rev. Mol. Cell Biol. 13, 370–382 (2012).
Marston, A.L. & Amon, A. Meiosis: cell-cycle controls shuffle and deal. Nat. Rev. Mol. Cell Biol. 5, 983–997 (2004).
Scherthan, H. A bouquet makes ends meet. Nat. Rev. Mol. Cell Biol. 2, 621–627 (2001).
Scherthan, H. et al. Centromere and telomere movements during early meiotic prophase of mouse and man are associated with the onset of chromosome pairing. J. Cell Biol. 134, 1109–1125 (1996).
Hiraoka, Y. & Dernburg, A.F. The SUN rises on meiotic chromosome dynamics. Dev. Cell 17, 598–605 (2009).
Sato, A. et al. Cytoskeletal forces span the nuclear envelope to coordinate meiotic chromosome pairing and synapsis. Cell 139, 907–919 (2009).
Conrad, M.N. et al. Rapid telomere movement in meiotic prophase is promoted by NDJ1, MPS3, and CSM4 and is modulated by recombination. Cell 133, 1175–1187 (2008).
Koszul, R., Kim, K.P., Prentiss, M., Kleckner, N. & Kameoka, S. Meiotic chromosomes move by linkage to dynamic actin cables with transduction of force through the nuclear envelope. Cell 133, 1188–1201 (2008).
Scherthan, H. et al. Chromosome mobility during meiotic prophase in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 104, 16934–16939 (2007).
Trelles-Sticken, E., Adelfalk, C., Loidl, J. & Scherthan, H. Meiotic telomere clustering requires actin for its formation and cohesin for its resolution. J. Cell Biol. 170, 213–223 (2005).
Ding, X. et al. SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev. Cell 12, 863–872 (2007).
Chikashige, Y. et al. Meiotic proteins bqt1 and bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell 125, 59–69 (2006).
Conrad, M.N., Dominguez, A.M. & Dresser, M.E. Ndj1p, a meiotic telomere protein required for normal chromosome synapsis and segregation in yeast. Science 276, 1252–1255 (1997).
Shibuya, H., Ishiguro, K. & Watanabe, Y. The TRF1-binding protein TERB1 promotes chromosome movement and telomere rigidity in meiosis. Nat. Cell Biol. 16, 145–156 (2014).
Shibuya, H. et al. MAJIN links telomeric DNA to the nuclear membrane by exchanging telomere cap. Cell 163, 1252–1266 (2015).
Shibuya, H. & Watanabe, Y. The meiosis-specific modification of mammalian telomeres. Cell Cycle 13, 2024–2028 (2014).
Chen, Y. et al. A shared docking motif in TRF1 and TRF2 used for differential recruitment of telomeric proteins. Science 319, 1092–1096 (2008).
Blazer, L.L., Roman, D.L., Muxlow, M.R. & Neubig, R.R. Use of flow cytometric methods to quantify protein-protein interactions. Current Protoc. Cytom. 51, 13.11 (2010).
Li, B., Oestreich, S. & de Lange, T. Identification of human Rap1: implications for telomere evolution. Cell 101, 471–483 (2000).
Arat, N.O. & Griffith, J.D. Human Rap1 interacts directly with telomeric DNA and regulates TRF2 localization at the telomere. J. Biol. Chem. 287, 41583–41594 (2012).
Frescas, D. & de Lange, T. Binding of TPP1 protein to TIN2 protein is required for POT1a,b protein-mediated telomere protection. J. Biol. Chem. 289, 24180–24187 (2014).
Takai, K.K., Kibe, T., Donigian, J.R., Frescas, D. & de Lange, T. Telomere protection by TPP1/POT1 requires tethering to TIN2. Mol. Cell 44, 647–659 (2011).
Banani, S.F., Lee, H.O., Hyman, A.A. & Rosen, M.K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Takai, K.K., Hooper, S., Blackwood, S., Gandhi, R. & de Lange, T. In vivo stoichiometry of shelterin components. J. Biol. Chem. 285, 1457–1467 2010).
Janoušková, E. et al. Human Rap1 modulates TRF2 attraction to telomeric DNA. Nucleic Acids Res. 43, 2691–2700 (2015).
Mossessova, E. & Lima, C.D. Ulp1-SUMO crystal structure and genetic analysis reveal conserved interactions and a regulatory element essential for cell growth in yeast. Mol. Cell 5, 865–876 (2000).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Cobb, J., Cargile, B. & Handel, M.A. Acquisition of competence to condense metaphase I chromosomes during spermatogenesis. Dev. Biol. 205, 49–64 (1999).
Shibuya, H., Morimoto, A. & Watanabe, Y. The dissection of meiotic chromosome movement in mice using an in vivo electroporation technique. PLoS Genet. 10, e1004821 (2014).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Acknowledgements
We thank the Life Sciences Collaborative Access Team at Argonne National Laboratory beamline staff for help with X-ray data collection, M. Iyer for help with protein purifications, and S. Grill for excellent feedback on the manuscript. This work was funded by NIH Grants R00CA167644 (to J.N.), R01GM120094 (to J.N.), R01AG050509 (to J.N., co-investigator), American Cancer Society Research Scholar grant 130882-RSG-17-037-01-DMC (to J.N.), NIH Biology of Aging Training Grant (T32AG000114) awarded to the University of Michigan Geriatrics Center from the National Institute on Aging (fellowship to E.M.S.), and MEXT KAKENHI 25000014 (to Y.W.).
Author information
Authors and Affiliations
Contributions
J.N., D.F.P., V.M.T., and Y.W. designed experiments; D.F.P., V.M.T., E.M.S., and J.N. conducted the crystallographic experiments; D.F.P. conducted cloning, protein purifications, and in vitro binding studies; Y.F. conducted in vivo cellular localization studies with guidance from H.S.; J.N., D.F.P., and Y.W. analyzed the data; J.N. and D.F.P. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 The TRF1-TERB1 interface.
(a) Overlay of the two TRF1TRFH-TERB1TRFB complexes observed in the dimeric crystal structure. The two TRFH domains are shown in cartoon depiction (green and cyan), while the two TBM peptides are shown in stick representation (orange and pink). (b) 2Fo – Fc map of the core of the TRF1-TERB1 interface contoured at 1.0 σ.
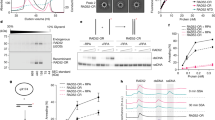
Supplementary Figure 2 Mutations in the TRF1-TERB1 interface abrogate binding.
(a) GST pull down of His-Smt3-TERB1TBM WT and mutant proteins using GST-TRF1TRFH as bait. Quantitation at the bottom was performed by dividing the appropriate band intensities by the molecular weight of the species followed by normalization against the molecular weight-normalized GST-TRF1TRFH signal in that line. (b) GST pull down of TRF1TRFH (WT or F142A) using GST-TERB1TBM as bait.
Supplementary Figure 3 Competition data for TERB1 TBM mutants with TRF1TRFH.
(a) And (b) are independent experimental replicates of data shown in Fig. 1j and k, respectively. Mean of technical duplicate is plotted. Mean and s.e.m. of biological duplicates (of technical duplicates) are shown in Table 2. (c) Size-exclusion (Superdex 200) profile of the TERB1TRFB and TERB21-107 complex following Ni-affinity purification and removal of the His-Smt3 tag. (d) Coomassie-stained SDS-PAGE for WT and indicated TERB1 mutant TERB1TRFB - TERB21-107 complexes eluted by size-exclusion chromatography. Yellow boxes highlight fractions indicative of a 1:1 TERB1:TERB2 complex. (e) Molecular weights of WT and TERB1 mutant TERB1TRFB - TERB21-107 complexes were determined by size-exclusion chromatography (Superdex 75). The theoretical molecular weight of a homodimer of the TERB1TRFB - TERB21-107 heterodimer (in kDa) along with the experimentally determined molecular weights (in kDa) is shown in a tabular format. (f) Equal amounts (5 μg) of indicated, purified TERB1TRFB - TERB21-107 complexes were analyzed using coomassie-stained SDS-PAGE.
Supplementary Figure 4 Cap exchange is not prevented in WT spermatocytes expressing TERB1 mutants that disrupt the TRF1-TERB1 interface.
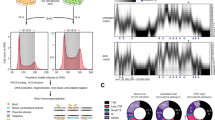
(a) Schematic depiction of TRF1 and TERB1 signal width and localization in early and late pachytene. While the TERB1 signal does not undergo a significant change in width throughout pachytene, the TRF1 signal becomes more diffuse resulting in an increase in its outer diameter. (b) WT late pachytene spermatocytes expressing GFP-TERB1 L647E, GFP-TERB1 T648D, GFP-TERB1 P649E, or GFP-TERB1 R651E-R652E (green) stained with TRF1 (red) and SYCP3 (blue) antibodies.
Supplementary Figure 5 Quantitation of anomalous TRF1 E93K and TERB1 I645F cap exchange.
(a) WT mouse spermatocytes expressing GFP-TRF1 E93K (green) stained with TRF1 (red) antibody in late pachytene. (b and c) Quantitation shows that GFP-TERB1 I645F distributes to the wider area compared to WT TERB1 protein. “n” and P-values indicated on graph in panel b.
Supplementary Figure 6 Composition and stoichiometry of the TRF1-TIN2 and TRF1-TERB1 complexes.
(a) Fluorescence-based competition experiments using Alexa Fluor 488-labeled GST-TIN2TBM pre-bound to biotin-tagged TRF1TRFH on streptavidin beads titrated with varying concentrations of unlabeled full-length TIN2 and TERB1TRFB – TERB21-107 proteins. Mean of technical duplicate is plotted. Error bars indicate s.e.m. (b) Size-exclusion (Superdex 200) profiles confirming intramolecular dimerization of TRFH-TRFH fusions (based on molecular weight determination). (c) Coomassie-stained SDS-PAGE for TRFH-TRFH intramolecular fusion proteins eluted by size-exclusion chromatography. (d) Replicate of experiment shown in Fig. 4c with quantitation shown at the bottom. (e) Mean and s.e.m. of duplicate experiments from panel d and Fig. 4c.
Supplementary Figure 7 Binding of TERB1 to TRF2 in vitro.
(a) Direct binding curves of biotin-tagged TRF1TRFH and TRF2TRFH with Alexa Fluor 488-labeled GST-TERB1TBM. Mean of technical duplicate is plotted. Mean and s.e.m. of duplicate measurements are indicated. (b) Competition experiments demonstrating similar affinity of TRF1 and TRF2 for TERB1TBM peptide. Mean of technical duplicate is plotted. Error bars indicate s.e.m. (c-d) Fluorescence-based competition experiments using Alexa Fluor 488-labeled GST-TERB1TBM pre-bound to biotin-tagged TRF2TRFH on streptavidin beads titrated with varying concentrations of indicated unlabeled TERB1TBM peptides (c) or TERB1TRFB – TERB21-107 complexes (d). For c and d, mean of technical duplicate is plotted and two biological replicates were performed. (e) Ternary pull down on amylose resin showing that TRF2 associates with MBP-tagged TERB1TRFB – TERB21-107 in the absence of Rap1 but not in its presence. (f) Ternary pull down on amylose resin showing that TRF2 associates with MBP-tagged TERB1TRFB – TERB21-107 in the presence or absence of TIN2.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Table 1 (PDF 1434 kb)
Rights and permissions
About this article
Cite this article
Pendlebury, D., Fujiwara, Y., Tesmer, V. et al. Dissecting the telomere–inner nuclear membrane interface formed in meiosis. Nat Struct Mol Biol 24, 1064–1072 (2017). https://doi.org/10.1038/nsmb.3493
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3493
This article is cited by
-
The S100A7 nuclear interactors in autoimmune diseases: a coevolutionary study in mammals
Immunogenetics (2022)
-
The SUN1-SPDYA interaction plays an essential role in meiosis prophase I
Nature Communications (2021)
-
The TERB1-TERB2-MAJIN complex of mouse meiotic telomeres dates back to the common ancestor of metazoans
BMC Evolutionary Biology (2020)
-
Structural biology of telomeres and telomerase
Cellular and Molecular Life Sciences (2020)
-
The meiotic TERB1-TERB2-MAJIN complex tethers telomeres to the nuclear envelope
Nature Communications (2019)