Abstract
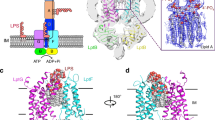
After biosynthesis, bacterial lipopolysaccharides (LPS) are transiently anchored to the outer leaflet of the inner membrane (IM). The ATP-binding cassette (ABC) transporter LptB2FG extracts LPS molecules from the IM and transports them to the outer membrane. Here we report the crystal structure of nucleotide-free LptB2FG from Pseudomonas aeruginosa. The structure reveals that lipopolysaccharide transport proteins LptF and LptG each contain a transmembrane domain (TMD), a periplasmic β-jellyroll-like domain and a coupling helix that interacts with LptB on the cytoplasmic side. The LptF and LptG TMDs form a large outward-facing V-shaped cavity in the IM. Mutational analyses suggest that LPS may enter the central cavity laterally, via the interface of the TMD domains of LptF and LptG, and is expelled into the β-jellyroll-like domains upon ATP binding and hydrolysis by LptB. These studies suggest a mechanism for LPS extraction by LptB2FG that is distinct from those of classical ABC transporters that transport substrates across the IM.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Raetz, C.R. & Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700 (2002).
Ruiz, N., Kahne, D. & Silhavy, T.J. Transport of lipopolysaccharide across the cell envelope: the long road of discovery. Nat. Rev. Microbiol. 7, 677–683 (2009).
Tan, Y. & Kagan, J.C. A cross-disciplinary perspective on the innate immune responses to bacterial lipopolysaccharide. Mol. Cell 54, 212–223 (2014).
Bryant, C.E., Spring, D.R., Gangloff, M. & Gay, N.J. The molecular basis of the host response to lipopolysaccharide. Nat. Rev. Microbiol. 8, 8–14 (2010).
Mühlradt, P.F. & Golecki, J.R. Asymmetrical distribution and artifactual reorientation of lipopolysaccharide in the outer membrane bilayer of Salmonella typhimurium . Eur. J. Biochem. 51, 343–352 (1975).
Karow, M. & Georgopoulos, C. The essential Escherichia coli msbA gene, a multicopy suppressor of null mutations in the htrB gene, is related to the universally conserved family of ATP-dependent translocators. Mol. Microbiol. 7, 69–79 (1993).
Eckford, P.D. & Sharom, F.J. The reconstituted Escherichia coli MsbA protein displays lipid flippase activity. Biochem. J. 429, 195–203 (2010).
Zhou, Z., White, K.A., Polissi, A., Georgopoulos, C. & Raetz, C.R. Function of Escherichia coli MsbA, an essential ABC family transporter, in lipid A and phospholipid biosynthesis. J. Biol. Chem. 273, 12466–12475 (1998).
Marolda, C.L., Tatar, L.D., Alaimo, C., Aebi, M. & Valvano, M.A. Interplay of the Wzx translocase and the corresponding polymerase and chain length regulator proteins in the translocation and periplasmic assembly of lipopolysaccharide o antigen. J. Bacteriol. 188, 5124–5135 (2006).
Abeyrathne, P.D., Daniels, C., Poon, K.K., Matewish, M.J. & Lam, J.S. Functional characterization of WaaL, a ligase associated with linking O-antigen polysaccharide to the core of Pseudomonas aeruginosa lipopolysaccharide. J. Bacteriol. 187, 3002–3012 (2005).
Ruiz, N., Gronenberg, L.S., Kahne, D. & Silhavy, T.J. Identification of two inner-membrane proteins required for the transport of lipopolysaccharide to the outer membrane of Escherichia coli . Proc. Natl. Acad. Sci. USA 105, 5537–5542 (2008).
Freinkman, E., Okuda, S., Ruiz, N. & Kahne, D. Regulated assembly of the transenvelope protein complex required for lipopolysaccharide export. Biochemistry 51, 4800–4806 (2012).
Villa, R. et al. The Escherichia coli Lpt transenvelope protein complex for lipopolysaccharide export is assembled via conserved structurally homologous domains. J. Bacteriol. 195, 1100–1108 (2013).
Freinkman, E., Chng, S.S. & Kahne, D. The complex that inserts lipopolysaccharide into the bacterial outer membrane forms a two-protein plug-and-barrel. Proc. Natl. Acad. Sci. USA 108, 2486–2491 (2011).
Sperandeo, P., Dehò, G. & Polissi, A. The lipopolysaccharide transport system of Gram-negative bacteria. Biochim. Biophys. Acta 1791, 594–602 (2009).
Narita, S. & Tokuda, H. Biochemical characterization of an ABC transporter LptBFGC complex required for the outer membrane sorting of lipopolysaccharides. FEBS Lett. 583, 2160–2164 (2009).
Sherman, D.J. et al. Decoupling catalytic activity from biological function of the ATPase that powers lipopolysaccharide transport. Proc. Natl. Acad. Sci. USA 111, 4982–4987 (2014).
Sperandeo, P. et al. Functional analysis of the protein machinery required for transport of lipopolysaccharide to the outer membrane of Escherichia coli . J. Bacteriol. 190, 4460–4469 (2008).
Bowyer, A., Baardsnes, J., Ajamian, E., Zhang, L. & Cygler, M. Characterization of interactions between LPS transport proteins of the Lpt system. Biochem. Biophys. Res. Commun. 404, 1093–1098 (2011).
Okuda, S., Freinkman, E. & Kahne, D. Cytoplasmic ATP hydrolysis powers transport of lipopolysaccharide across the periplasm in E. coli . Science 338, 1214–1217 (2012).
Tran, A.X., Dong, C. & Whitfield, C. Structure and functional analysis of LptC, a conserved membrane protein involved in the lipopolysaccharide export pathway in Escherichia coli . J. Biol. Chem. 285, 33529–33539 (2010).
Suits, M.D., Sperandeo, P., Dehò, G., Polissi, A. & Jia, Z. Novel structure of the conserved gram-negative lipopolysaccharide transport protein A and mutagenesis analysis. J. Mol. Biol. 380, 476–488 (2008).
Sperandeo, P. et al. Characterization of lptA and lptB, two essential genes implicated in lipopolysaccharide transport to the outer membrane of Escherichia coli . J. Bacteriol. 189, 244–253 (2007).
Chng, S.S., Ruiz, N., Chimalakonda, G., Silhavy, T.J. & Kahne, D. Characterization of the two-protein complex in Escherichia coli responsible for lipopolysaccharide assembly at the outer membrane. Proc. Natl. Acad. Sci. USA 107, 5363–5368 (2010).
Chimalakonda, G. et al. Lipoprotein LptE is required for the assembly of LptD by the beta-barrel assembly machine in the outer membrane of Escherichia coli . Proc. Natl. Acad. Sci. USA 108, 2492–2497 (2011).
Chng, S.S. et al. Disulfide rearrangement triggered by translocon assembly controls lipopolysaccharide export. Science 337, 1665–1668 (2012).
Malojcˇic´, G. et al. LptE binds to and alters the physical state of LPS to catalyze its assembly at the cell surface. Proc. Natl. Acad. Sci. USA 111, 9467–9472 (2014).
Ruiz, N., Chng, S.S., Hiniker, A., Kahne, D. & Silhavy, T.J. Nonconsecutive disulfide bond formation in an essential integral outer membrane protein. Proc. Natl. Acad. Sci. USA 107, 12245–12250 (2010).
Wu, T. et al. Identification of a protein complex that assembles lipopolysaccharide in the outer membrane of Escherichia coli . Proc. Natl. Acad. Sci. USA 103, 11754–11759 (2006).
Qiao, S., Luo, Q., Zhao, Y., Zhang, X.C. & Huang, Y. Structural basis for lipopolysaccharide insertion in the bacterial outer membrane. Nature 511, 108–111 (2014).
Dong, H. et al. Structural basis for outer membrane lipopolysaccharide insertion. Nature 511, 52–56 (2014).
Botos, I. et al. Structural and functional characterization of the LPS transporter LptDE from Gram-negative pathogens. Structure 24, 965–976 (2016).
Hvorup, R.N. et al. Asymmetry in the structure of the ABC transporter-binding protein complex BtuCD-BtuF. Science 317, 1387–1390 (2007).
Oldham, M.L., Khare, D., Quiocho, F.A., Davidson, A.L. & Chen, J. Crystal structure of a catalytic intermediate of the maltose transporter. Nature 450, 515–521 (2007).
Hollenstein, K., Frei, D.C. & Locher, K.P. Structure of an ABC transporter in complex with its binding protein. Nature 446, 213–216 (2007).
Dawson, R.J. & Locher, K.P. Structure of the multidrug ABC transporter Sav1866 from Staphylococcus aureus in complex with AMP-PNP. FEBS Lett. 581, 935–938 (2007).
Hohl, M., Briand, C., Grütter, M.G. & Seeger, M.A. Crystal structure of a heterodimeric ABC transporter in its inward-facing conformation. Nat. Struct. Mol. Biol. 19, 395–402 (2012).
Okuda, S., Sherman, D.J., Silhavy, T.J., Ruiz, N. & Kahne, D. Lipopolysaccharide transport and assembly at the outer membrane: the PEZ model. Nat. Rev. Microbiol. 14, 337–345 (2016).
Simpson, B.W. et al. Identification of residues in the lipopolysaccharide ABC transporter that coordinate ATPase activity with extractor function. MBio 7, e01729–16 (2016).
Lee, J.Y. et al. Crystal structure of the human sterol transporter ABCG5/ABCG8. Nature 533, 561–564 (2016).
Lizak, C., Gerber, S., Numao, S., Aebi, M. & Locher, K.P. X-ray structure of a bacterial oligosaccharyltransferase. Nature 474, 350–355 (2011).
Bai, X.C., Rajendra, E., Yang, G., Shi, Y. & Scheres, S.H. Sampling the conformational space of the catalytic subunit of human γ-secretase. eLife 4, e11182 (2015).
Sherman, D.J., Okuda, S., Denny, W.A. & Kahne, D. Validation of inhibitors of an ABC transporter required to transport lipopolysaccharide to the cell surface in Escherichia coli. Bioorg. Med. Chem. 21, 4846–4851 (2013).
Wang, Z. et al. Structural and functional studies of conserved nucleotide-binding protein LptB in lipopolysaccharide transport. Biochem. Biophys. Res. Commun. 452, 443–449 (2014).
Lin, D.Y., Huang, S. & Chen, J. Crystal structures of a polypeptide processing and secretion transporter. Nature 523, 425–430 (2015).
Ward, A.B. et al. Structures of P-glycoprotein reveal its conformational flexibility and an epitope on the nucleotide-binding domain. Proc. Natl. Acad. Sci. USA 110, 13386–13391 (2013).
Yakushi, T., Masuda, K., Narita, S., Matsuyama, S. & Tokuda, H. A new ABC transporter mediating the detachment of lipid-modified proteins from membranes. Nat. Cell Biol. 2, 212–218 (2000).
Scheich, C., Kümmel, D., Soumailakakis, D., Heinemann, U. & Büssow, K. Vectors for co-expression of an unrestricted number of proteins. Nucleic Acids Res. 35, e43 (2007).
Wang, Q.S. et al. The macromolecular crystallography beamline of SSRF. Nucl. Sci. Tech. 26, 12–17 (2015).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Adams, P.D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 (2002).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
DeLano, W.L. PyMOL molecular viewer: updates and refinements. in Abstracts of Papers of the American Chemical Society 238 (ACS, 2009).
Acknowledgements
The authors thank Z. Liu, X. Zhang and H. Wu for valuable discussions and critically reading the manuscript. The authors also thank N. Ruiz (Department of Microbiology, The Ohio State University, Columbus, Ohio, USA) for generously providing the lptFG-depleted NR1113 E. coli strain. The diffraction data were collected at the Shanghai Synchrotron Radiation Facility (SSRF, China) and National Center for Protein Science Shanghai (NCPSS, China). This work was supported by grants from the National Natural Science Foundation of China (31625009 to Y.H.), the Ministry of Science and Technology (2016YFA0500404 and 2013CB910603 to Y.H.) and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB08020302 to Y. H.).
Author information
Authors and Affiliations
Contributions
Y.H. supervised the project. Q.L., X.Y., S.Y., H.S., K.W., L.X. and T.L. performed the experiments. Q.L. and Y.H. collected diffraction data. Y.H., Q.L. and G.Z. built the model and refined the structure. Y.H., C.S., G.Z., X.Z., D.L. and M.Z. contributed to manuscript preparation. Y.H. and Q.L. wrote the manuscript. All authors contributed to data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Chemical structure of LPS and LPS biogenesis in Gram-negative bacteria.
a. Chemical structure of LPS. LPS molecule consists of Lipid A, core oligosaccharide and O-antigen. The polar part of Lipid A is negatively-changed due to the presence of two phosphate groups. b. Ra-LPS molecule is approximately 32Å in height and 28 Å×12 Å in the other two dimensions. The dimensions of an Ra-LPS is based on the crystal structure of TLR4/MD-2/Ra-LPS complex (PDB ID: 3FXI). c. LPS biogenesis in Gram-negative bacteria. After flipped to the IM outer leaflet by MsbA, LPS is extracted from IM, transported cross the periplasm and finally inserted in the OM by the LptABCDEFG transenvelope complex. LptB2FG is a quaternary ABC transporter.
Supplementary Figure 2 The electron density maps of LptB2FG.
a. Stereo views (cross-eyed) of the 2Fo-Fc electron density map for the complete LptB2FG complex structure at 3.46Å. b. The 2Fo-Fc electron density maps of representative regions of the TMDs of LptFG (TM1-LptF and TM1-LptG) are shown. Selenomethionine residues (in red) and bulky residues are used as makers for guiding model building. c-d. Validation of side-chain register of the nucleotide-free LptB2FG transporter. Anomalous electron density maps define selenomethionine (contour level: 3.0σ) in (c) and Pt sites (contour level: 4.5σ) in (d). In (c), anomalous density was observed for 28 out of 32 selenomethionines of the complete LptB2FG complex.
Supplementary Figure 3 Domain organization of the LptB2FG complex.
a. Domain organization of the LptB2FG complex. The two ATPase domains (LptB) in cytoplasm are colored in cyan and green. The TMD domains of LptF and LptG, each containing six transmembrane helices, are colored in violet and yellow, respectively. The two periplasmic β-jellyroll domains of LptF and LptG that stem from TM3 and TM4 of LptF(G) are colored in grey. The two coupling helices of LptF and LptG connecting TM2 and TM3 of LptF(G) in cytoplasm are highlighted in blue. b. Overlay of the TMD of LptF with that of LptG. LptF and LptG are colored in violet and yellow, respectively.
Supplementary Figure 4 Sequence alignment of LptF homologs and residues selected for functional analysis in the structure.
a. Sequence alignment of LptF homologs from five representative Gram-negative bacterial strains. b. Conserved hydrophobic and positive residues of LptF lining the “V”-shaped cavity selected for mutational studies. Conservation of LptF residues in different Gram-negative homologs is shown in ENDscript. Secondary structures are numbered within the respective domains. Conserved residues lining the inner surface of the “V”-shaped cavity were selected for mutational analyses are highlighted. The labeled residue types and numbers in both alignments correspond to those in E. coli.
Supplementary Figure 5 Sequence alignment of LptG homologs and residues selected for functional analysis in the structure.
a. Sequence alignment of LptG homologs from five representative Gram-negative bacterial strains. b. Conserved hydrophobic and positive residues of LptG lining the “V”-shaped cavity selected for mutational studies. Conservation of LptG residues in different Gram-negative homologs is shown in ENDscript. Secondary structures are numbered within the respective domains. Conserved residues lining the inner surface of the “V”-shaped cavity were selected for mutational analyses are highlighted. The labeled residue types and numbers in both alignments correspond to those in E. coli.
Supplementary Figure 6 Mutagenesis study of the conserved residues that line the inner surface of the “V”-shaped cavity in the TMDs of LptF and LptG.
The growth phenotypes of the lptFG-depleted E. coli strain NR1113 transformed with various hydrophobic-to-hydrophilic LptG_Ec mutants (a) and LptF_Ec mutants (b) on LB agar plates containing 0.1% L-arabinose and 50 μg ml−1 kanamycin. The growth phenotypes of the lptFG-depleted E. coli strain NR1113 transformed with positive-to-negative mutations in the absence of L-aribinose (c), mutant protein expression levels (d) and the growth phenotypes in the presence of 0.1% L-arabinose (e). Mutational analyses of residues from the coupling helices of LptF_Ec and LptG_Ec on LB agar plates containing 0.1% L-arabinose and 50 μg ml−1 kanamycin (f). All labeled residue types and numbers correspond to those of LptF_Ec and LptG_Ec. In the presence of 0.1% L-arabinose, the lptFG-depleted E. coli strain NR1113 transformed with WT, vector control and various mutants all grew well similar to that of WT. In the absence of L-arabinose, the lptFG-depleted E. coli strain NR1113 transformed with pQLink-Kan vector and plasmids encoding wild-type (WT) LptFG were used as negative control and positive control, respectively. All the complementation assays were repeated at least three times and a representative result is shown.
Supplementary Figure 7 Three representative ABC exporters in their inward-facing or outward-facing conformational states.
The nucleotide-free LptB2FG transporter (a), the heterodimeric TM287-TM288 exporter in the apo state (PDB code: 4Q4H) (b) and the heterodimeric nucleotide-free human sterol ABCG5-ABCG8 exporter (PDB code: 5DO7) (c) in inward-facing conformational state; the homodimeric AMP-PNP-bound Sav1866 exporter (PDB code: 2ONJ) (d) in outward-facing conformational state.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 1557 kb)
Rights and permissions
About this article
Cite this article
Luo, Q., Yang, X., Yu, S. et al. Structural basis for lipopolysaccharide extraction by ABC transporter LptB2FG. Nat Struct Mol Biol 24, 469–474 (2017). https://doi.org/10.1038/nsmb.3399
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3399
This article is cited by
-
Genome-wide association study reveals serovar-associated genetic loci in Riemerella anatipestifer
BMC Genomics (2024)
-
Conformational Investigation of the Asymmetric Periplasmic Domains of E. coli LptB2FGC Using SDSL CW EPR Spectroscopy
Applied Magnetic Resonance (2024)
-
Structure of an endogenous mycobacterial MCE lipid transporter
Nature (2023)
-
Structural basis for triacylglyceride extraction from mycobacterial inner membrane by MFS transporter Rv1410
Nature Communications (2023)
-
Antibacterial effects assessment on some livestock pathogens, thermal stability and proposing a probable reason for different levels of activity of thanatin
Scientific Reports (2021)