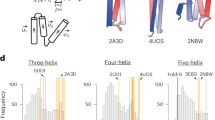
This Perspective provides an overview of the major advances in recent years in the computational design and structure prediction of α-helical membrane proteins.
Abstract
The computational design of α-helical membrane proteins is still in its infancy but has already made great progress. De novo design allows stable, specific and active minimal oligomeric systems to be obtained. Computational reengineering can improve the stability and function of naturally occurring membrane proteins. Currently, the major hurdle for the field is the experimental characterization of the designs. The emergence of new structural methods for membrane proteins will accelerate progress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Li, Z., Yang, Y., Zhan, J., Dai, L. & Zhou, Y. Energy functions in de novo protein design: current challenges and future prospects. Annu. Rev. Biophys. 42, 315–335 (2013).
Pantazes, R.J., Grisewood, M.J. & Maranas, C.D. Recent advances in computational protein design. Curr. Opin. Struct. Biol. 21, 467–472 (2011).
Richardson, J.S. & Richardson, D.C. The de novo design of protein structures. Trends Biochem. Sci. 14, 304–309 (1989).
Ambroggio, X.I. & Kuhlman, B. Computational design of a single amino acid sequence that can switch between two distinct protein folds. J. Am. Chem. Soc. 128, 1154–1161 (2006).
Kuhlman, B. et al. Design of a novel globular protein fold with atomic-level accuracy. Science 302, 1364–1368 (2003).
Jiang, L. et al. De novo computational design of retro-aldol enzymes. Science 319, 1387–1391 (2008).
Röthlisberger, D. et al. Kemp elimination catalysts by computational enzyme design. Nature 453, 190–195 (2008).
Siegel, J.B. et al. Computational design of an enzyme catalyst for a stereoselective bimolecular Diels-Alder reaction. Science 329, 309–313 (2010).
Khare, S.D. et al. Computational redesign of a mononuclear zinc metalloenzyme for organophosphate hydrolysis. Nat. Chem. Biol. 8, 294–300 (2012).
Krogh, A., Larsson, B., von Heijne, G. & Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305, 567–580 (2001).
Almén, M.S., Nordström, K.J.V., Fredriksson, R. & Schiöth, H.B. Mapping the human membrane proteome: a majority of the human membrane proteins can be classified according to function and evolutionary origin. BMC Biol. 7, 50 (2009).
Liu, J. & Rost, B. Comparing function and structure between entire proteomes. Protein Sci. 10, 1970–1979 (2001).
Korendovych, I.V. et al. De novo design and molecular assembly of a transmembrane diporphyrin-binding protein complex. J. Am. Chem. Soc. 132, 15516–15518 (2010).
Joh, N.H. et al. De novo design of a transmembrane Zn2+-transporting four-helix bundle. Science 346, 1520–1524 (2014).
Forrest, L.R. Structural symmetry in membrane proteins. Annu. Rev. Biophys. 44, 311–337 (2015).
Grigoryan, G. Absolute free energies of biomolecules from unperturbed ensembles. J. Comput. Chem. 34, 2726–2741 (2013).
Hallen, M.A., Keedy, D.A. & Donald, B.R. Dead-end elimination with perturbations (DEEPer): a provable protein design algorithm with continuous sidechain and backbone flexibility. Proteins 81, 18–39 (2013).
Morrison, E.A. et al. Antiparallel EmrE exports drugs by exchanging between asymmetric structures. Nature 481, 45–50 (2012).
Senes, A., Gerstein, M. & Engelman, D.M. Statistical analysis of amino acid patterns in transmembrane helices: the GxxxG motif occurs frequently and in association with beta-branched residues at neighboring positions. J. Mol. Biol. 296, 921–936 (2000).
Russ, W.P. & Engelman, D.M. The GxxxG motif: a framework for transmembrane helix-helix association. J. Mol. Biol. 296, 911–919 (2000).
Walters, R.F.S. & DeGrado, W.F. Helix-packing motifs in membrane proteins. Proc. Natl. Acad. Sci. USA 103, 13658–13663 (2006).
Zhang, S.-Q. et al. The membrane- and soluble-protein helix-helix interactome: similar geometry via different interactions. Structure 23, 527–541 (2015).
Senes, A., Engel, D.E. & DeGrado, W.F. Folding of helical membrane proteins: the role of polar, GxxxG-like and proline motifs. Curr. Opin. Struct. Biol. 14, 465–479 (2004).
Senes, A., Ubarretxena-Belandia, I. & Engelman, D.M. The Cα—H⋯O hydrogen bond: a determinant of stability and specificity in transmembrane helix interactions. Proc. Natl. Acad. Sci. USA 98, 9056–9061 (2001).
Feng, X. & Barth, P. A topological and conformational stability alphabet for multipass membrane proteins. Nat. Chem. Biol. 12, 167–173 (2016).
Yin, H. et al. Computational design of peptides that target transmembrane helices. Science 315, 1817–1822 (2007).
Mueller, B.K., Subramaniam, S. & Senes, A. A frequent, GxxxG-mediated, transmembrane association motif is optimized for the formation of interhelical Cα-H hydrogen bonds. Proc. Natl. Acad. Sci. USA 111, E888–E895 (2014).
Mori, T., Miyashita, N., Im, W., Feig, M. & Sugita, Y. Molecular dynamics simulations of biological membranes and membrane proteins using enhanced conformational sampling algorithms. Biochim. Biophys. Acta http://dx.doi.org/10.1016/j.bbamem.2015.12.032 (2016).
Cheng, Y., Grigorieff, N., Penczek, P.A. & Walz, T. A primer to single-particle cryo-electron microscopy. Cell 161, 438–449 (2015).
Bai, X.C., McMullan, G. & Scheres, S.H.W. How cryo-EM is revolutionizing structural biology. Trends Biochem. Sci. 40, 49–57 (2015).
Poulos, S., Morgan, J.L.W., Zimmer, J. & Faham, S. Bicelles coming of age: an empirical approach to bicelle crystallization. Methods Enzymol. 557, 393–416 (2015).
Williamson, J.A. et al. Structure and multistate function of the transmembrane electron transporter CcdA. Nat. Struct. Mol. Biol. 22, 809–814 (2015).
Wang, S. et al. Solid-state NMR spectroscopy structure determination of a lipid-embedded heptahelical membrane protein. Nat. Methods 10, 1007–1012 (2013).
Isogai, S. et al. Backbone NMR reveals allosteric signal transduction networks in the β1-adrenergic receptor. Nature 530, 237–241 (2016).
Manglik, A. et al. Structural insights into the dynamic process of β2-adrenergic receptor signaling. Cell 161, 1101–1111 (2015).
Liu, J.J., Horst, R., Katritch, V., Stevens, R.C. & Wüthrich, K. Biased signaling pathways in β2-adrenergic receptor characterized by 19F-NMR. Science 335, 1106–1110 (2012).
Zhou, Y. & Bowie, J.U. Building a thermostable membrane protein. J. Biol. Chem. 275, 6975–6979 (2000).
Katritch, V., Cherezov, V. & Stevens, R.C. Structure-function of the G protein-coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 53, 531–556 (2013).
Magnani, F., Shibata, Y., Serrano-Vega, M.J. & Tate, C.G. Co-evolving stability and conformational homogeneity of the human adenosine A2a receptor. Proc. Natl. Acad. Sci. USA 105, 10744–10749 (2008).
Sarkar, C.A. et al. Directed evolution of a G protein-coupled receptor for expression, stability, and binding selectivity. Proc. Natl. Acad. Sci. USA 105, 14808–14813 (2008).
Chun, E. et al. Fusion partner toolchest for the stabilization and crystallization of G protein-coupled receptors. Structure 20, 967–976 (2012).
Egloff, P. et al. Structure of signaling-competent neurotensin receptor 1 obtained by directed evolution in Escherichia coli . Proc. Natl. Acad. Sci. USA 111, E655–E662 (2014).
Chen, K.-Y.M., Zhou, F., Fryszczyn, B.G. & Barth, P. Naturally evolved G protein-coupled receptors adopt metastable conformations. Proc. Natl. Acad. Sci. USA 109, 13284–13289 (2012).
Vaidehi, N., Grisshammer, R. & Tate, C.G. How can mutations thermostabilize G-protein-coupled receptors? Trends Pharmacol. Sci. 37, 37–46 (2016).
Conklin, B.R. et al. Engineering GPCR signaling pathways with RASSLs. Nat. Methods 5, 673–678 (2008).
Roth, B.L. DREADDs for neuroscientists. Neuron 89, 683–694 (2016).
Knight, Z.A. & Shokat, K.M. Features of selective kinase inhibitors. Chem. Biol. 12, 621–637 (2005).
Tinberg, C.E. et al. Computational design of ligand-binding proteins with high affinity and selectivity. Nature 501, 212–216 (2013).
Bhattacharya, S. & Vaidehi, N. Differences in allosteric communication pipelines in the inactive and active states of a GPCR. Biophys. J. 107, 422–434 (2014).
Miao, Y., Nichols, S.E., Gasper, P.M., Metzger, V.T. & McCammon, J.A. Activation and dynamic network of the M2 muscarinic receptor. Proc. Natl. Acad. Sci. USA 110, 10982–10987 (2013).
LeVine, M.V. & Weinstein, H. NbIT: a new information theory-based analysis of allosteric mechanisms reveals residues that underlie function in the leucine transporter LeuT. PLoS Comput. Biol. 10, e1003603 (2014).
Yarov-Yarovoy, V., Schonbrun, J. & Baker, D. Multipass membrane protein structure prediction using Rosetta. Proteins 62, 1010–1025 (2006).
Barth, P., Schonbrun, J. & Baker, D. Toward high-resolution prediction and design of transmembrane helical protein structures. Proc. Natl. Acad. Sci. USA 104, 15682–15687 (2007).
Barth, P., Wallner, B. & Baker, D. Prediction of membrane protein structures with complex topologies using limited constraints. Proc. Natl. Acad. Sci. USA 106, 1409–1414 (2009).
Hopf, T.A. et al. Three-dimensional structures of membrane proteins from genomic sequencing. Cell 149, 1607–1621 (2012).
Ovchinnikov, S. et al. Large-scale determination of previously unsolved protein structures using evolutionary information. eLife 4, e09248 (2015).
Wang, Y. & Barth, P. Evolutionary-guided de novo structure prediction of self-associated transmembrane helical proteins with near-atomic accuracy. Nat. Commun. 6, 7196 (2015).
Chen, K.-Y.M., Sun, J., Salvo, J.S., Baker, D. & Barth, P. High-resolution modeling of transmembrane helical protein structures from distant homologues. PLoS Comput. Biol. 10, e1003636 (2014).
Eswar, N., Eramian, D., Webb, B., Shen, M.-Y. & Sali, A. Protein structure modeling with MODELLER. Methods Mol. Biol. 426, 145–159 (2008).
Roy, A., Kucukural, A. & Zhang, Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5, 725–738 (2010).
Kelm, S., Shi, J. & Deane, C.M. MEDELLER: homology-based coordinate generation for membrane proteins. Bioinformatics 26, 2833–2840 (2010).
Zhang, J., Yang, J., Jang, R. & Zhang, Y. GPCR-I-TASSER: a hybrid approach to G protein-coupled receptor structure modeling and the application to the human genome. Structure 23, 1538–1549 (2015).
Bhattacharya, S. et al. Critical analysis of the successes and failures of homology models of G protein-coupled receptors. Proteins 81, 729–739 (2013).
Mandell, D.J., Coutsias, E.A. & Kortemme, T. Sub-angstrom accuracy in protein loop reconstruction by robotics-inspired conformational sampling. Nat. Methods 6, 551–552 (2009).
Tang, K., Zhang, J. & Liang, J. Fast protein loop sampling and structure prediction using distance-guided sequential chain-growth Monte Carlo method. PLoS Comput. Biol. 10, e1003539 (2014).
Acknowledgements
P.B. is supported by National Institutes of Health grant R01GM097207, by a Lilly Research Award Program and by a supercomputer allocation from XSEDE (MCB120101). A.S. is supported by National Institutes of Health grant R01GM0997522 and National Science Foundation grant CHE-1415910. We are grateful to S. Condon, S. Anderson, X. Feng and H. Cao for critical reading of the manuscript and to G. Grigoryan for providing the model of Rocker.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Barth, P., Senes, A. Toward high-resolution computational design of the structure and function of helical membrane proteins. Nat Struct Mol Biol 23, 475–480 (2016). https://doi.org/10.1038/nsmb.3231
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3231
This article is cited by
-
Small-residue packing motifs modulate the structure and function of a minimal de novo membrane protein
Scientific Reports (2020)
-
The First 3D Model of the Full-Length KIT Cytoplasmic Domain Reveals a New Look for an Old Receptor
Scientific Reports (2020)
-
Helical polymers for biological and medical applications
Nature Reviews Chemistry (2020)
-
The de novo design of a biocompatible and functional integral membrane protein using minimal sequence complexity
Scientific Reports (2018)