Abstract
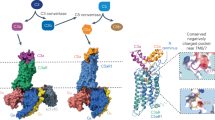
Activation of complement C5 generates the potent anaphylatoxin C5a and leads to pathogen lysis, inflammation and cell damage. The therapeutic potential of C5 inhibition has been demonstrated by eculizumab, one of the world's most expensive drugs. However, the mechanism of C5 activation by C5 convertases remains elusive, thus limiting development of therapeutics. Here we identify and characterize a new protein family of tick-derived C5 inhibitors. Structures of C5 in complex with the new inhibitors, the phase I and phase II inhibitor OmCI, or an eculizumab Fab reveal three distinct binding sites on C5 that all prevent activation of C5. The positions of the inhibitor-binding sites and the ability of all three C5–inhibitor complexes to competitively inhibit the C5 convertase conflict with earlier steric-inhibition models, thus suggesting that a priming event is needed for activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
Primary accessions
Biological Magnetic Resonance Data Bank
Electron Microscopy Data Bank
NCBI Reference Sequence
Protein Data Bank
Referenced accessions
GenBank/EMBL/DDBJ
Protein Data Bank
References
Kemper, C. & Köhl, J. Novel roles for complement receptors in T cell regulation and beyond. Mol. Immunol. 56, 181–190 (2013).
Ricklin, D., Hajishengallis, G., Yang, K. & Lambris, J.D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797 (2010).
Law, S.K. & Dodds, A.W. The internal thioester and the covalent binding properties of the complement proteins C3 and C4. Protein Sci. 6, 263–274 (1997).
Coulthard, L.G. & Woodruff, T.M. Is the complement activation product C3a a proinflammatory molecule? Re-evaluating the evidence and the myth. J. Immunol. 194, 3542–3548 (2015).
Pangburn, M.K. & Rawal, N. Structure and function of complement C5 convertase enzymes. Biochem. Soc. Trans. 30, 1006–1010 (2002).
Rawal, N. & Pangburn, M. Formation of high-affinity C5 convertases of the alternative pathway of complement. J. Immunol. 166, 2635–2642 (2001).
Rawal, N. & Pangburn, M.K. Formation of high affinity C5 convertase of the classical pathway of complement. J. Biol. Chem. 278, 38476–38483 (2003).
Daha, M.R., Fearon, D.T. & Austen, K.F. C3 requirements for formation of alternative pathway C5 convertase. J. Immunol. 117, 630–634 (1976).
Medicus, R.G., Schreiber, R.D., Götze, O. & Müller-Eberhard, H.J. A molecular concept of the properdin pathway. Proc. Natl. Acad. Sci. USA 73, 612–616 (1976).
Woodruff, T.M., Nandakumar, K.S. & Tedesco, F. Inhibiting the C5-C5a receptor axis. Mol. Immunol. 48, 1631–1642 (2011).
Morgan, B.P. The membrane attack complex as an inflammatory trigger. Immunobiology doi:10.1016/j.imbio.2015.04.006 (30 April 2015).
Markiewski, M.M. & Lambris, J.D. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 171, 715–727 (2007).
Ricklin, D. & Lambris, J.D. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J. Immunol. 190, 3831–3838 (2013).
Mollnes, T.E. & Kirschfink, M. Strategies of therapeutic complement inhibition. Mol. Immunol. 43, 107–121 (2006).
Nunn, M.A. et al. Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata. J. Immunol. 174, 2084–2091 (2005).
Nene, V. et al. Genes transcribed in the salivary glands of female Rhipicephalus appendiculatus ticks infected with Theileria parva. Insect Biochem. Mol. Biol. 34, 1117–1128 (2004).
Barratt-Due, A. et al. Ornithodoros moubata complement inhibitor is an equally effective C5 inhibitor in pigs and humans. J. Immunol. 187, 4913–4919 (2011).
Hepburn, N.J., Chamberlain-Banoub, J.L., Williams, A.S., Morgan, B.P. & Harris, C.L. Prevention of experimental autoimmune myasthenia gravis by rat Crry-Ig: a model agent for long-term complement inhibition in vivo. Mol. Immunol. 45, 395–405 (2008).
Fredslund, F. et al. Structure of and influence of a tick complement inhibitor on human complement component 5. Nat. Immunol. 9, 753–760 (2008).
Laursen, N.S. et al. Substrate recognition by complement convertases revealed in the C5-cobra venom factor complex. EMBO J. 30, 606–616 (2011).
Laursen, N.S. et al. Structural basis for inhibition of complement C5 by the SSL7 protein from Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 107, 3681–3686 (2010).
Zhang, X., Boyar, W., Galakatos, N. & Gonnella, N.C. Solution structure of a unique C5a semi-synthetic antagonist: implications in receptor binding. Protein Sci. 6, 65–72 (1997).
Kini, R.M. & Doley, R. Structure, function and evolution of three-finger toxins: mini proteins with multiple targets. Toxicon 56, 855–867 (2010).
Mans, B.J. & Ribeiro, J.M. Function, mechanism and evolution of the moubatin-clade of soft tick lipocalins. Insect Biochem. Mol. Biol. 38, 841–852 (2008).
Roversi, P. et al. Bifunctional lipocalin ameliorates murine immune complex-induced acute lung injury. J. Biol. Chem. 288, 18789–18802 (2013).
Roversi, P. et al. The structure of OMCI, a novel lipocalin inhibitor of the complement system. J. Mol. Biol. 369, 784–793 (2007).
Nishimura, J. et al. Genetic variants in C5 and poor response to eculizumab. N. Engl. J. Med. 370, 632–639 (2014).
Thomas, T.C. et al. Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol. Immunol. 33, 1389–1401 (1996).
Elmlund, D. & Elmlund, H. Cryogenic electron microscopy and single-particle analysis. Annu. Rev. Biochem. 84, 499–517 (2015).
Janssen, B.J. et al. Insights into complement convertase formation based on the structure of the factor B-cobra venom factor complex. EMBO J. 28, 2469–2478 (2009).
Mortensen, S. et al. Structural basis for the function of complement component C4 within the classical and lectin pathways of complement. J. Immunol. 194, 5488–5496 (2015).
Rooijakkers, S.H. et al. Structural and functional implications of the alternative complement pathway C3 convertase stabilized by a staphylococcal inhibitor. Nat. Immunol. 10, 721–727 (2009).
Janssen, B.J., Christodoulidou, A., McCarthy, A., Lambris, J.D. & Gros, P. Structure of C3b reveals conformational changes that underlie complement activity. Nature 444, 213–216 (2006).
Reis, E.S. et al. Applying complement therapeutics to rare diseases. Clin. Immunol. 161, 225–240 (2015).
Vogt, W., Schmidt, G., Von Buttlar, B. & Dieminger, L. A new function of the activated third component of complement: binding to C5, an essential step for C5 activation. Immunology 34, 29–40 (1978).
Kinoshita, T. et al. C5 convertase of the alternative complement pathway: covalent linkage between two C3b molecules within the trimolecular complex enzyme. J. Immunol. 141, 3895–3901 (1988).
Takata, Y. et al. Covalent association of C3b with C4b within C5 convertase of the classical complement pathway. J. Exp. Med. 165, 1494–1507 (1987).
Berends, E.T. et al. Molecular insights into the surface-specific arrangement of complement C5 convertase enzymes. BMC Biol. 13, 93 (2015).
Rawal, N. & Pangburn, M.K. Functional role of the noncatalytic subunit of complement C5 convertase. J. Immunol. 164, 1379–1385 (2000).
Sandoval, A., Ai, R., Ostresh, J.M. & Ogata, R.T. Distal recognition site for classical pathway convertase located in the C345C/netrin module of complement component C5. J. Immunol. 165, 1066–1073 (2000).
Grabherr, M.G. et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29, 644–652 (2011).
Roos, A. et al. Functional characterization of the lectin pathway of complement in human serum. Mol. Immunol. 39, 655–668 (2003).
Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995).
Güntert, P. Automated NMR structure calculation with CYANA. Methods Mol. Biol. 278, 353–378 (2004).
Tian, Y., Opella, S.J. & Marassi, F.M. Improved chemical shift prediction by Rosetta conformational sampling. J. Biomol. NMR 54, 237–243 (2012).
McCoy, A.J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Winn, M.D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Adams, P.D. et al. Advances, interactions, and future developments in the CNS, Phenix, and Rosetta structural biology software systems. Annu. Rev. Biophys. 42, 265–287 (2013).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
Elmlund, H., Elmlund, D. & Bengio, S. PRIME: probabilistic initial 3D model generation for single-particle cryo-electron microscopy. Structure 21, 1299–1306 (2013).
Elmlund, D. & Elmlund, H. SIMPLE: software for ab initio reconstruction of heterogeneous single-particles. J. Struct. Biol. 180, 420–427 (2012).
Yang, Z. et al. UCSF Chimera, MODELLER, and IMP: an integrated modeling system. J. Struct. Biol. 179, 269–278 (2012).
Acknowledgements
We thank N. Darvill, T. Tang and L. Britton for experimental contributions, S. Jensen for technical support, M. Slovak (Institute of Zoology, Bratislava, Slovakia) for providing salivary glands, D.R. Greaves (University of Oxford) for mouse serum and T.E. Mollnes (Oslo University Hospital) for pig serum. We thank the High-Throughput Genomics Group at the Wellcome Trust Centre for Human Genetics (funded by Wellcome Trust grant reference 090532/Z/09/Z and Medical Research Council Hub grant G0900747 91070) for generating the sequencing data. This work was financially supported by a Rubicon grant from the Netherlands Organisation for Scientific Research to M.M.J. (825.11.030) and a Wellcome Investigator Award to S.M.L. (100298). We thank the staff at the Clive and Vera Ramaciotti Centre for Structural Cryo-Electron Microscopy at Monash University for assistance with EM image acquisition. We acknowledge the Diamond Light Source for time on I02 under proposal MX9306. EM calculations were performed within the Multi-modal Australian Sciences Imaging and Visualisation Environment (MASSIVE).
Author information
Authors and Affiliations
Contributions
S.M.L. and M.A.N. initially conceived the work. S.M.L. and M.M.J. designed the biochemical experiments, and M.M.J. performed them with assistance from N.M.B. M.M.J. crystallized the complexes, and the structures were solved by S.M.L. and S.J. M.M.J., S.M.L. and S.J. analyzed the structures. D.S. designed, collected and solved the NMR structure. H.E. designed the EM experiments and, together with S.M.L., collected and analyzed the data. Y.I.L. processed the HiSeq data and assembled the transcriptome. All authors were involved in discussion of results and preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
M.M.J., M.A.N. and S.M.L. hold intellectual property in the area of use of recombinant tick complement inhibitors as therapeutic agents.
Integrated supplementary information
Supplementary Figure 1 Complement inhibition by salivary gland extract (SGE) and RaCI homologs.
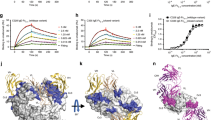
(A) SGE from both male and female ticks inhibit the classical pathway in a haemolysis assay. SGE equalling 2 glands (female) or 1 gland (male) were added to the haemolysis assay. Error bars, s.e.m. (n = 3 technical replicates). (B) Inhibition of classical pathway in a haemolysis assay with supernatants from stable transfected Drosophila melanogaster S2 cell lines. Individual values of two technical replicates are shown. (C) Classical and alternative pathway inhibition by RaCI family members and OmCI using an ELISA based activation assay, similar to the Wieslab assay used in Fig. 1b. The difference between the IC50 values for CP and AP inhibition likely reflects the difference in C5 concentrations used in the assays (1% serum in CP assay versus 10% serum in the AP assay). Error bars, s.e.m. (n = 3 technical replicates). (D) Cross-species reactivity of RaCI homologues and OmCI in a haemolysis assay. Error bars, s.e.m. (n = 3 technical replicates).
Supplementary Figure 2 The custom-made Fab (EcuFab), based on the sequence of Eculizumab, is a fully active complement inhibitor.
The custom-made Fab (EcuFab), based on the sequence of Eculizumab, is a fully active complement inhibitor. Classical (CP) and alternative (AP) pathway inhibition by the Fab fragment using an ELISA based activation assay show that the IC50 values are similar to RaCIs and OmCI in Supplementary Figure 1. Error bars, s.e.m. (n = 3 technical replicates).
Supplementary Figure 3 Different location of C345c domain in our new structures of inhibited C5 compared with apo-C5.
Different location of C345c domain with respect to the rest of C5 in our new structures of inhibited C5 (wheat) compared to the earlier structures of apo-C5 (orange; Fredslund et al., Nat. Immunol. 9, 753-760, 2011). The new location is consistent with that previously seen in the CVF-C5 complex (olive; Laursen et al., EMBO J. 9,606-616, 2011). The CVF component of the C5-CVF complex is shown in a salmon ribbon representation highlighting the clash with the C345c domain in the apo-C5 like position. The residues that will form the anaphylatoxin C5a are coloured in shades of red and the overall view is the same as in the close-up of this region shown in Figure 3. The structures are overlaid by superposition of the C5a domain in each structure.
Supplementary Figure 4 Overviews of the ternary complex.
(A) Front view of C5-OmCI-RaCI3 complex. (B) Cartoon showing domain organisation of C5 and interaction sites for both tick-inhibitors (yellow stars)
Supplementary Figure 5 RaCI contacts mapped onto cross-species sequence alignment of the MG1 and MG2 domains of C5.
C5 residues that make contact with one or more RaCIs in the crystal structures are highlighted in black (van der Waals interaction) and red (salt or hydrogen bonds).
Supplementary Figure 6 RaCI contacts mapped onto cross-species sequence alignment of the C5d domain of C5.
C5 residues that make contact with one or more RaCIs in the crystal structures are highlighted in black (van der Waals interaction) and red (salt or hydrogen bonds). The large deletion in the Canis lupus sequence is likely due to sequencing or assembly errors and may not represent the actual sequence.
Supplementary Figure 7 OmCI contacts mapped onto cross-species sequence alignment of the CUB and C5d domains of C5.
C5 residues that make contact with OmCI in the crystal structures are highlighted in black (van der Waals interaction) and red (salt or hydrogen bonds). The large deletion in the Canis lupus sequence is likely due to sequencing or assembly errors and may not represent the actual sequence.
Supplementary Figure 8 OmCI contacts mapped onto cross-species sequence alignment of the C345c domain of C5.
C5 residues that make contact with OmCI in the crystal structures are highlighted in black (van der Waals interaction) and red (salt or hydrogen bonds).
Supplementary Figure 9 OmCI can bind C5 and LTB4 simultaneously.
An overlay of the structure of OmCI (blue cartoon) in complex with LTB4 (VDW spheres, carbon-green, oxygen-red) (PDB ID 3zuo; Roversi et al., J. Biol. Chem 288, 18789-18802, 2013) onto OmCI (cyan cartoon) in complex with C5 (grey cartoon) demonstrates that LTB4 binding and exchange are both compatible with C5 binding. Two views related by a rotation of 180 degrees are shown with the view on the right hand side being equivalent to the views of the complex shown in Fig. 2.
Supplementary Figure 10 The binary inhibited C5 complexes are competitive inhibitors of further C5 cleavage by the native convertase.
Purified binary C5 complexes with any of OmCI, RaCI or EcuFab compete with C5 at an initial stage of the activation pathway. –ve control is histamine-binding protein 2. Error bars, s.e.m. (n = 6; 2 independent experiments with 3 technical replicates each).
Supplementary Figure 11 Eculizumab sterically hinders the binding of CVF to C5.
Crystal structure of C5-OmCI-RaCI fitted in the EM envelope as in Figure 5E. Superposition of C5-CVF complex (Laursen et al., EMBO J. 9,606-616, 2011) on the C5-OmCI-RaCI structure results in a steric clash between CVF (salmon ribbon) and the EM volume where the Fab is positioned.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Supplementary Tables 1 and 2 (PDF 6806 kb)
Rights and permissions
About this article
Cite this article
Jore, M., Johnson, S., Sheppard, D. et al. Structural basis for therapeutic inhibition of complement C5. Nat Struct Mol Biol 23, 378–386 (2016). https://doi.org/10.1038/nsmb.3196
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3196
This article is cited by
-
Advancing Treatment in Bullous Pemphigoid: A Comprehensive Review of Novel Therapeutic Targets and Approaches
Clinical Reviews in Allergy & Immunology (2023)
-
Structure and function of a family of tick-derived complement inhibitors targeting properdin
Nature Communications (2022)
-
Effects of early pregnancy on the complement system in the ovine thymus
Veterinary Research Communications (2022)
-
Dual inhibition of complement component 5 and leukotriene B4 by topical rVA576 in atopic keratoconjunctivis: TRACKER phase 1 clinical trial results
Orphanet Journal of Rare Diseases (2021)
-
Alpha-synuclein activates the classical complement pathway and mediates complement-dependent cell toxicity
Journal of Neuroinflammation (2021)