Abstract
Mig6 is a feedback inhibitor that directly binds, inhibits and drives internalization of ErbB-family receptors. Mig6 selectively targets activated receptors. Here we found that the epidermal growth factor receptor (EGFR) phosphorylates Mig6 on Y394 and that this phosphorylation is primed by prior phosphorylation of an adjacent residue, Y395, by Src. Crystal structures of human EGFR–Mig6 complexes reveal the structural basis for enhanced phosphorylation of primed Mig6 and show how Mig6 rearranges after phosphorylation by EGFR to effectively irreversibly inhibit the same receptor that catalyzed its phosphorylation. This dual phosphorylation site allows Mig6 to inactivate EGFR in a manner that requires activation of the target receptor and that can be modulated by Src. Loss of Mig6 is a driving event in human cancer; analysis of 1,057 gliomas reveals frequent focal deletions of ERRFI1, the gene that encodes Mig6, in EGFR-amplified glioblastomas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Fiorentino, L. et al. Inhibition of ErbB-2 mitogenic and transforming activity by RALT, a mitogen-induced signal transducer which binds to the ErbB-2 kinase domain. Mol. Cell. Biol. 20, 7735–7750 (2000).
Hackel, P.O., Gishizky, M. & Ullrich, A. Mig-6 is a negative regulator of the epidermal growth factor receptor signal. Biol. Chem. 382, 1649–1662 (2001).
Xu, D., Makkinje, A. & Kyriakis, J.M. Gene 33 is an endogenous inhibitor of epidermal growth factor (EGF) receptor signaling and mediates dexamethasone-induced suppression of EGF function. J. Biol. Chem. 280, 2924–2933 (2005).
Segatto, O., Anastasi, S. & Alema, S. Regulation of epidermal growth factor receptor signalling by inducible feedback inhibitors. J. Cell Sci. 124, 1785–1793 (2011).
Fiorini, M. et al. Expression of RALT, a feedback inhibitor of ErbB receptors, is subjected to an integrated transcriptional and post-translational control. Oncogene 21, 6530–6539 (2002).
Zhang, Y.W. & Vande Woude, G.F. Mig-6, signal transduction, stress response and cancer. Cell Cycle 6, 507–513 (2007).
Anastasi, S., Baietti, M.F., Frosi, Y., Alema, S. & Segatto, O. The evolutionarily conserved EBR module of RALT/MIG6 mediates suppression of the EGFR catalytic activity. Oncogene 26, 7833–7846 (2007).
Zhang, X. et al. Inhibition of the EGF receptor by binding of MIG6 to an activating kinase domain interface. Nature 450, 741–744 (2007).
Descot, A. et al. Negative regulation of the EGFR-MAPK cascade by actin-MAL-mediated Mig6/Errfi-1 induction. Mol. Cell 35, 291–304 (2009).
Frosi, Y. et al. A two-tiered mechanism of EGFR inhibition by RALT/MIG6 via kinase suppression and receptor degradation. J. Cell Biol. 189, 557–571 (2010).
Ying, H. et al. Mig-6 controls EGFR trafficking and suppresses gliomagenesis. Proc. Natl. Acad. Sci. USA 107, 6912–6917 (2010).
Ferby, I. et al. Mig6 is a negative regulator of EGF receptor-mediated skin morphogenesis and tumor formation. Nat. Med. 12, 568–573 (2006).
Zhang, Y.W. et al. Evidence that MIG-6 is a tumor-suppressor gene. Oncogene 26, 269–276 (2007).
Maity, T.K. et al. Loss of MIG6 accelerates initiation and progression of mutant epidermal growth factor receptor-driven lung adenocarcinoma. Cancer Discov (2015).
Anastasi, S. et al. Loss of RALT/MIG-6 expression in ERBB2-amplified breast carcinomas enhances ErbB-2 oncogenic potency and favors resistance to Herceptin. Oncogene 24, 4540–4548 (2005).
Makkinje, A. et al. Gene 33/Mig-6, a transcriptionally inducible adapter protein that binds GTP-Cdc42 and activates SAPK/JNK. A potential marker transcript for chronic pathologic conditions, such as diabetic nephropathy: possible role in the response to persistent stress. J. Biol. Chem. 275, 17838–17847 (2000).
Anastasi, S. et al. Feedback inhibition by RALT controls signal output by the ErbB network. Oncogene 22, 4221–4234 (2003).
Wang, Z. et al. Mechanistic insights into the activation of oncogenic forms of EGF receptor. Nat. Struct. Mol. Biol. 18, 1388–1393 (2011).
Lemmon, M.A. & Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134 (2010).
Kandoth, C. et al. Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 (2013).
Brennan, C.W. et al. The somatic genomic landscape of glioblastoma. Cell 155, 462–477 (2013).
Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 511, 543–550 (2014).
Sharma, S.V., Bell, D.W., Settleman, J. & Haber, D.A. Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer 7, 169–181 (2007).
Mok, T.S. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361, 947–957 (2009).
Yu, H.A. & Pao, W. Targeted therapies: afatinib: new therapy option for EGFR-mutant lung cancer. Nat. Rev. Clin. Oncol. 10, 551–552 (2013).
Chong, C.R. & Janne, P.A. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat. Med. 19, 1389–1400 (2013).
Lee, J.C. et al. Epidermal growth factor receptor activation in glioblastoma through novel missense mutations in the extracellular domain. PLoS Med. 3, e485 (2006).
Francis, J.M. et al. EGFR variant heterogeneity in glioblastoma resolved through single-nucleus sequencing. Cancer Discov. 4, 956–971 (2014).
Wong, A.J. et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc. Natl. Acad. Sci. USA 89, 2965–2969 (1992).
Verhaak, R.G. et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17, 98–110 (2010).
Guha, U. et al. Comparisons of tyrosine phosphorylated proteins in cells expressing lung cancer-specific alleles of EGFR and KRAS. Proc. Natl. Acad. Sci. USA 105, 14112–14117 (2008).
Wolf-Yadlin, A. et al. Effects of HER2 overexpression on cell signaling networks governing proliferation and migration. Mol. Syst. Biol. 2, 54 (2006).
Wang, Z. et al. Tyrosine phosphorylation of mig6 reduces its inhibition of the epidermal growth factor receptor. ACS Chem. Biol. 8, 2372–2376 (2013).
Ficarro, S.B. et al. Magnetic bead processor for rapid evaluation and optimization of parameters for phosphopeptide enrichment. Anal. Chem. 81, 4566–4575 (2009).
Guo, A. et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc. Natl. Acad. Sci. USA 105, 692–697 (2008).
Cho, J. et al. Glioblastoma-derived epidermal growth factor receptor carboxyl-terminal deletion mutants are transforming and are sensitive to EGFR-directed therapies. Cancer Res. 71, 7587–7596 (2011).
Greulich, H. et al. Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS Med. 2, e313 (2005).
Yasuda, H. et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci. Transl. Med. 5, 216ra177 (2013).
Galisteo, M.L., Yang, Y., Urena, J. & Schlessinger, J. Activation of the nonreceptor protein tyrosine kinase Ack by multiple extracellular stimuli. Proc. Natl. Acad. Sci. USA 103, 9796–9801 (2006).
Shen, F., Lin, Q., Gu, Y., Childress, C. & Yang, W. Activated Cdc42-associated kinase 1 is a component of EGF receptor signaling complex and regulates EGF receptor degradation. Mol. Biol. Cell 18, 732–742 (2007).
Muir, T.W., Sondhi, D. & Cole, P.A. Expressed protein ligation: a general method for protein engineering. Proc. Natl. Acad. Sci. USA 95, 6705–6710 (1998).
Silverman, R.B. Mechanism-based enzyme inactivators. Methods Enzymol. 249, 240–283 (1995).
Carpentier, J.L., White, M.F., Orci, L. & Kahn, R.C. Direct visualization of the phosphorylated epidermal growth factor receptor during its internalization in A-431 cells. J. Cell Biol. 105, 2751–2762 (1987).
Bose, R., Holbert, M.A., Pickin, K.A. & Cole, P.A. Protein tyrosine kinase-substrate interactions. Curr. Opin. Struct. Biol. 16, 668–675 (2006).
Bettegowda, C. et al. Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science 333, 1453–1455 (2011).
Zack, T.I. et al. Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 45, 1134–1140 (2013).
Hopkins, S. et al. Mig6 is a sensor of EGF receptor inactivation that directly activates c-Abl to induce apoptosis during epithelial homeostasis. Dev. Cell 23, 547–559 (2012).
Bagchi, A. & Mills, A.A. The quest for the 1p36 tumor suppressor. Cancer Res. 68, 2551–2556 (2008).
Red Brewer, M. et al. Mechanism for activation of mutated epidermal growth factor receptors in lung cancer. Proc. Natl. Acad. Sci. USA 110, E3595–E3604 (2013).
Cho, J. et al. Cetuximab response of lung cancer-derived EGF receptor mutants is associated with asymmetric dimerization. Cancer Res. 73, 6770–6779 (2013).
Adelmant, G. et al. in Sample Preparation in Biological Mass Spectrometry (eds. Ivanov A.R. & Lazarev, A.V.) 437–486 (Springer, 2011).
Ficarro, S.B. et al. Improved electrospray ionization efficiency compensates for diminished chromatographic resolution and enables proteomics analysis of tyrosine signaling in embryonic stem cells. Anal. Chem. 81, 3440–3447 (2009).
Parikh, J.R. et al. multiplierz: an extensible API based desktop environment for proteomics data analysis. BMC Bioinformatics 10, 364 (2009).
Yuza, Y. et al. Allele-dependent variation in the relative cellular potency of distinct EGFR inhibitors. Cancer Biol. Ther. 6, 661–667 (2007).
Yun, C.H. et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell 11, 217–227 (2007).
Otwinowski, Z., Borek, D., Majewski, W. & Minor, W. Multiparametric scaling of diffraction intensities. Acta Crystallogr. A 59, 228–234 (2003).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Cancer Genome Atlas Research Network. et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N. Engl. J. Med. 372, 2481–2498 (2015).
Acknowledgements
We thank L. Cantley and M. Begley for helpful discussions and sharing unpublished data, and R. McNally for critical reading of the manuscript. We thank P. Cole, D. Leahy and Z. Wang for helpful discussions and for testing the effect of phospho-Mig6 species on the activity of transmembrane EGFR (Supplementary Fig. 4e). We thank P. Janne (Dana-Farber Cancer Institute) for providing cells. We thank beamline personnel at the Northeast Collaborative Access Team at the Advanced Photon Source, Argonne National Laboratory (NE-CAT) for assistance with data collection and processing. NE-CAT is supported by grants from the US National Institutes of Health (NIH). This work was supported in part by NIH grants CA116020 (M.J.E. and M.M.), CA154303 (M.J.E. and M.M.), CA156021 (J.A.M.) and CA178860 (J.A.M.). This work was also supported in part by grants from Samsung Medical Center (J.C.) and the Basic Science Research Program of the National Research Foundation of Korea, Ministry of Science, ICT and Future Planning (grant 2013065771; J.C.).
Author information
Authors and Affiliations
Contributions
E.P. designed and conducted all in vitro biochemical and enzyme kinetic studies and determined all crystal structures. N.K., A.C., K.K., A.K.J.P. and W.-Y.P. designed and carried out cell-based functional studies of Mig6. J.A.M. conceived and supervised proteomics and other mass spectrometry–based experiments. S.B.F. and Y.Z. designed and executed mass spectrometry–based experiments. B.I.L. refined crystal structure 4ZJV. B.M. and R.B. designed and executed genomic analyses of ERRFI1. M.M., J.C. and M.J.E. conceived the study, designed experiments and, together with E.P., S.F., R.B. and J.A.M., wrote and edited the manuscript. All authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Quantitative phosphoproteomic workflow and identification of EGFR-driven phosphorylation in NIH-3T3 cells.
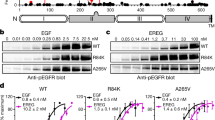
a, Analytical workflow leading to identification of Mig6 pY394/pY395 site. Tryptic peptides derived from NIH-3T3 cells expressing WT EGFR or various mutant EGFR (Ex20Ins, Ex20Ins/D837A (kinase-dead) or C-terminal deletion mutants) were iTRAQ-labeled (114-WT+EGF, 115-Ex20Ins, 116-Ex20Ins-KD, 117-CT DEL1) and subjected to enrichment and clean-up of phospho-tyrosine-containing peptides using anti-phospho-tyrosine antibodies followed by immobilized metal affinity chromatorgraphy IIMAC). Next, iTRAQ labeled peptides were analyzed by LC-MS/MS, with peptide sequence and phosphorylation site assignments based on the Mascot search algorithm. To complement previous phosphoproteomic studies that focused on the L858R and Ex19Del EGFR mutants, we studied an exon20 insertion mutant (insNPG) and an oncogenic C-terminal deletion mutant (CTDel1), which deletes residues 1010-1152). We included a kinase-dead (D837A) version of the exon20 insertion mutant as a negative control. b, Using this approach we identified a total of 640 phosphopeptides that mapped to 511 unique gene identifiers (≤1% false discovery rate, FDR). The histogram distribution of iTRAQ reporter ion Log-ratios for these 640 phosphopeptides are plotted. Selected phosphorylation sites listed on the right were upregulated at least 8-fold relative to the kinase-dead control in all three experiments (WT+EGF, Ex20Ins, and CTDel). This analysis revealed increased phosphorylation of EGFR itself in addition to adaptor proteins that mediate EGFR and Src signaling (including CrkL, Dok1, Gab3 and Shc1), as well as key downstream signaling proteins including STAT5b and Shp2.
Supplementary Figure 2 Validation and characterization of EGFR-driven phosphorylation of Mig6.
a and b, Characterization of phospho-specific Y394Y395 Mig6 antibody. Expression constructs of WT(a) or L858R or Ex20Ins EGFR mutants (b) were transiently transfected alone or in combination with either WT or Y394F/Y395F Mig6 mutants in HEK-293T cells. The resulting cell lysates were subjected to immunoblotting with an affinity purified rabbit polyclonal antibody raised against a phosphorylated Mig6 peptide (with sequence SSTHpYpYLLPER). The levels of ectopically expressed EGFR and Mig6 proteins were examined by immunoblotting with anti-EGFR or antiflag antibodies. Anti-β-actin was used as a loading control. c and d, EGFR phosphorylates Mig6 segment 1+2 predominately on Y394. Equimolar EGFR and Mig6 (330-399) were mixed under reaction conditions and the fraction of Mig6 in each phosphorylation state (at the Y394/Y395 site) was determined by quantitative mass spectrometry at the indicated time points. Parallel experiments were conducted with L858R mutant EGFR (c) and WT EGFR (d).
Supplementary Figure 3 Phosphorylation of Mig6 on Y394 is essential for binding and inhibition of WT and mutant EGFR.
a, Immunoblotting analysis was performed using cell lysates prepared from the NIH-3T3 cells stably expressing the indicated Mig6 and EGFR variants (corresponding to Fig. 3b in main text) with antibodies against EGFR, p-EGFR (4G10), Mig6 (flag) or p-Mig6. β-actin antibody was used as a loading control. b, Y394F and Y394F/Y395F mutations impair the ability of Mig6 to inhibit constitutive phosphorylation of L858R EGFR. Cell lysates derived from HEK-293T cells transiently cotransfected expression constructs of L858R EGFR with WT or mutant Mig6 as indicated were subjected to immunoblotting with antibodies against EGFR, p-EGFR (4G10), Mig6 (flag), p-Mig6 and β-actin. c, Binding of Mig6 to L858R EGFR is abrogated by erlotinib treatment and by mutation of the Y394/Y395 phosphorylation site. Cell lysates derived from HEK-293T cells transiently transfected with EGFR L858R and Mig6 alone or in combination as indicated were immunoprecipitated with antibodies against EGFR followed by immunoblotting analysis with anti-flag or anti-EGFR antibodies, respectively. Immunoblotting of whole cell lysates as indicated revealed equivalent levels of ectopically expressed EGFR and Mig6. β-actin antibody was used as a loading control.
Supplementary Figure 4 Preparation and characterization of semisynthetic Mig6 phosphoproteins.
a, Scheme for preparation of Mig6 segment 1+2 proteins (residues 330-399) in defined phosphorylation states using intein-mediated expressed protein ligation (EPL). Mig6 residues 330-389 were expressed in the chitin EPL vector pTBX1, modified to encode a glutathione-Stransferase (GST) fusion and a tobacco etch virus (TEV) protease site. Synthetic phosphopeptides corresponding to Mig6 residues 390-399, with Ser 390 replaced by cysteine, were ligated to the Mig6-330-389 fragment following MESNA cleavage (2-mercaptoethanesulfonic acid). Preparation of the doubly-phosphorylated Mig6-pYpY protein is illustrated, analogous procedues were used to prepare the singly-phosphorylated species. Note that this synthetic approach introduces a Ser390Cys substitution. b, Analysis of unphosphorylated Mig6 and semisynthetic Mig6 phosphorylation variants by Coomassie staining and western blotting with phosphospecific antibodies. Parallel SDS-PAGE gels were Coomassie-stained or immunoblotted with anti-phosphotyrosine antibody 4G10, anti-phospho-Mig6, or the anti-phospho-Mig6 further purified by subtractive purification with an immobilized
Mig6 pY394 peptide (anti-phospho-Mig6*). c, Inhibition of EGFR (L858R or wild type) by Mig6 segment 1+2 or semi-synthetic Mig6 segment 1+2 (residues 330-399) in defined phosphorylation states. The kinase was preincubated with increasing concentrations of the indicated Mig6 phosphorylation variant and reaction velocity was measured at steady state (30 minutes after starting the reaction by addition of ATP). Fractional velocities are plotted as a function of Mig6 concentration. Poly[E4Y] was used as a peptide substrate at a concentration of 2.5 mM. d, Inhibition of EGFR L858R by Mig6-YY versus Mig6-pYpY at moderate concentrations (0.16 mM) of peptide substrate poly[E4Y]. Enzyme activity was measured at steady state. Inhibition of L858R EGFR by Mig6-pYpY is well-fit by Morrison’s equation for tightbinding inhibitors (purple curve), but inhibition by the unphosphorylated Mig6-YY protein is not (blue curve). At low concentrations, Mig6-YY exhibits potent inhibition (up to 160 nM Mig6-YY), but inhibition effectively plateaus at higher concentrations. This effect arises from the fact that Mig6-YY is not the actual inhibitory species, and once EGFR is saturated with Mig6-YY (that it
has converted to Mig6-pYY or Mig6-pYpY), little additional phospho-Mig6 is produced. Thus at each point in the titration the reaction contains a mixture of unphosphorylated and phosphorylated Mig6. At low concentrations virtually all of the Mig6 is phosphorylated, while at higher concentrations only a small, nearly fixed amount of inhibitory phospho-Mig6 is present. EGFR L858R was used at a concentration of 100 nM active enzyme. e, Inhibition of EGF-bound, near full-length EGFR by Mig6 segment 1+2 or semi-synthetic Mig6 segment 1+2 (residues 330-399) in defined phosphorylation states. Radiometric kinase assays were performed as previously described by Wang et al. (ref. 33). Briefly, Mig6 was preincubated with 10 nM tEGFR on ice for 10 min before initiating the reaction with 30 μM peptide substrate (Biotin- RAHEEIYHFFFAKKK-COOH). After stopping the reaction, streptavidin Ultralink resin (Pierce) was used to isolate the biotinylated substrate. The resin was washed three times, and incorporated radioactivity was measured by liquid scintillation counting. Turnover was <10%. All reactions were performed in duplicate, which showed values that were generally within 20%.
Supplementary Figure 5 Binding studies of phosphorylated Mig6 (330–399) to EGFR.
a, Kinase-dead EGFR retains the ability to bind phosphorylated Mig6. An excess of semisynthetic Mig6-pYpY was incubated with EGFR D837N and was then analyzed by size-exclusion chromatography and SDS-PAGE. The elution profile (absorbance at 280 nm) is shown in the upper panel, aligned with Coomassie-stained SDS-PAGE of corresponding elution fractions (middle panel) and a western blot with the Mig6-pY394/pY395 specific antisera (lower panel). b, Dissociation of phosphorylated Mig6 (segment 1+2) from EGFR is extremely slow. A two-fold molar excess of EGFR L858R was combined with GST-Mig6 (330-399) and incubated for 20 minutes with or without ATP. The reaction mixture was captured on glutathione agarose beads, which were washed with continuous flow for the indicated time periods before elution with glutathione and analysis by SDS-Page. Note that binding is ATP-dependent, consistent with a requirement for phosphorylation on Y394. Using gel densitometry to measure the ratio of bound EGFR to GST-Mig6, we calculated a t1/2 of ~45 minutes for the complex.
Supplementary Figure 6 Crystal structures of EGFR in complex with Mig6.
a, Electron density map in the region of pY394/pY395 in Mig6-pYpY peptide complex. The 2Fo-Fc map (blue mesh) is contoured at 1.2σ. We also calculated a difference electron density map with coefficients [Fobs(pYpY)-Fobs(YpY)] and phases derived from the apo-EGFR model. The strongest feature in this completely unbiased difference map is a positive peak (> 8σ) in the position of the phosphate group on pTyr394 (green mesh, contoured at 4σ). b, Stereo view of electron density in Mig6 segment 1 + 2 structure. Map is contoured at 1.2σ in region of Mig6 segment 2. Mig6 is shown in yellow, EGFR in blue. Note hydrophobic packing of isoleucines 379 and 382.
Supplementary Figure 7 Enzyme kinetic studies of Mig6 inhibition of WT and mutant EGFR.
a, Mig6 is a peptide-substrate competitive inhibitor with Ki=15.85 nM. Apparent Ki values (Ki app) for Mig6- pYpY (residues 330-399) were determined at a range of poly[E4Y] concentrations; extrapolation to zero substrate concentration by linear least-squares fitting (red line) was used to determine the Ki value. b, EGFR L858R activity was measured at a range of poly[E4Y] concentrations in the presence of increasing concentrations of Mig6-pYpY. Apparent Km values (Kmapp) at each Mig6-pYpY concentration were determined by fitting to the Michaelis-Menten equation using Graphpad PRISM software, and were as follows: no Mig6, Kmapp = 243 μM; 0.04 μM Mig6, Kmapp = 402 μM; 0.16 μM Mig6, Kmapp = 1.29 mM; 0.64 μM Mig6, Kmapp = 2.0 mM, and 2.56 μM Mig6, Kmapp = 1.45 mM. The increase in Kmapp with increasing Mig6-pYpY concentrations indicates a peptide-substrate competitive mechanism of inhibition. c, Comparison of inhibition of WT, L858R (LR), and T790M/V948R (TMVR) EGFR proteins by Mig6-YY. The V948R mutation is on the C-lobe of EGFR in the Mig6 segment 1 binding site. This mutation decreases, but does not abolish inhibition by Mig6. (We expect that the T790M mutation is without effect; the double mutant was used here as we had previously prepared it for an unrelated study).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 1399 kb)
Supplementary Data Set 1
Gel and blot original images (PDF 3064 kb)
Supplementary Table 1
Quantitative phosphoproteomic data for WT and mutant EGFR signaling images (XLS 206 kb)
Rights and permissions
About this article
Cite this article
Park, E., Kim, N., Ficarro, S. et al. Structure and mechanism of activity-based inhibition of the EGF receptor by Mig6. Nat Struct Mol Biol 22, 703–711 (2015). https://doi.org/10.1038/nsmb.3074
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3074
This article is cited by
-
Systematic Profiling of Mitogen-Inducible Gene 6 and Its Derived Peptides Binding to Receptor Tyrosine Kinases in Bone Cancers at Molecular and Cellular Levels
International Journal of Peptide Research and Therapeutics (2024)
-
Coupled folding-upon-binding of human tumor suppressor MIG6 to lung cancer EGFR kinase domain and molecular trimming/stapling of MIG6-derived β-hairpins to target the coupling event
European Biophysics Journal (2023)
-
Molecular insight into the affinity, specificity and cross-reactivity of systematic hepatocellular carcinoma RALT interaction profile with human receptor tyrosine kinases
Amino Acids (2021)
-
Making ERRFI1-Derived Peptides ‘Bindable’ to the Allosteric Dimerization Interface of Breast Cancer ERBB3 Kinase by Adding a Nonbonded Interaction System
International Journal of Peptide Research and Therapeutics (2021)
-
Directed Molecular Engineering of Mig6 Peptide Selectivity between Proto-oncogene ErbB Family Receptor Tyrosine Kinases
Biotechnology and Bioprocess Engineering (2021)