Abstract
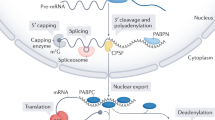
Polyadenylated mRNAs are typically more strongly repressed by microRNAs (miRNAs) than their nonadenylated counterparts. Using a Drosophila melanogaster cell-free translation system, we found that this effect is mediated by the poly(A)-binding protein (PABP). miRNA repression was positively correlated with poly(A) tail length, but this stimulatory effect on repression was lost when translation was repressed by the tethered GW182 silencing domain rather than the miRNA-induced silencing complex (miRISC) itself. These findings are mechanistically explained by a notable function of PABP: it promotes association of miRISC with miRNA-regulated mRNAs. We also found that PABP association with mRNA rapidly diminished with miRISC recruitment and before detectable deadenylation. We integrated these data into a revised model for the function of PABP and the poly(A) tail in miRNA-mediated translational repression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bushati, N. & Cohen, S.M. microRNA functions. Annu. Rev. Cell Dev. Biol. 23, 175–205 (2007).
Huntzinger, E. & Izaurralde, E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat. Rev. Genet. 12, 99–110 (2011).
Kahvejian, A., Roy, G. & Sonenberg, N. The mRNA closed-loop model: the function of PABP and PABP-interacting proteins in mRNA translation. Cold Spring Harb. Symp. Quant. Biol. 66, 293–300 (2001).
Fabian, M.R. et al. Mammalian miRNA RISC recruits CAF1 and PABP to affect PABP-dependent deadenylation. Mol. Cell 35, 868–880 (2009).
Mishima, Y. et al. Translational inhibition by deadenylation-independent mechanisms is central to microRNA-mediated silencing in zebrafish. Proc. Natl. Acad. Sci. USA 109, 1104–1109 (2012).
Zekri, L., Huntzinger, E., Heimstädt, S. & Izaurralde, E. The silencing domain of GW182 interacts with PABC1 to promote translational repression and degradation of miRNA targets and is required for target release. Mol. Cell. Biol. 29, 6220–6231 (2009).
Huntzinger, E., Braun, J.E., Heimstädt, S., Zekri, L. & Izaurralde, E. Two PABC1-binding sites in GW182 proteins promote miRNA-mediated gene silencing. EMBO J. 29, 4146–4160 (2010).
Jinek, M., Fabian, M.R., Coyle, A.M., Sonenberg, N. & Doudna, J. Structural insights into the human GW182-PABP interaction in microRNA-mediated deadenylation. Nat. Struct. Mol. Biol. 17, 238–240 (2010).
Kozlov, G., Safaee, N., Rosenauer, A. & Gehring, K. Structural basis of binding of P-body-associated proteins GW182 and Ataxin-2 by the Mlle domain of poly(A)-binding protein. J. Biol. Chem. 285, 13599–13606 (2010).
Eulalio, A., Tritschler, F. & Izaurralde, E. The GW182 protein family in animal cells: New insights into domains required for miR-mediated gene silencing. RNA 15, 1433–1442 (2009).
Braun, J.E., Huntzinger, E., Fauser, M. & Izaurralde, E. GW182 proteins directly recruit cytoplasmic deadenylase complexes to miRNA targets. Mol. Cell 44, 120–133 (2011).
Eulalio, A. et al. Deadenylation is a widespread effect of miRNA regulation. RNA 15, 21–32 (2009).
Fukaya, T. & Tomari, Y. PABP is not essential for microRNA-mediated translational repression and deadenylation in vitro. EMBO J. 30, 4998–5009 (2011).
Wu, L., Fan, J. & Belasco, J.G. MicroRNAs direct rapid deadenylation of mRNA. Proc. Natl. Acad. Sci. USA 103, 4034–4039 (2006).
Zdanowicz, A. et al. D. melanogaster miR2 primarily targets the m7GpppN cap structure for translational repression. Mol. Cell 35, 881–888 (2009).
Iwasaki, S., Kawamata, T. & Tomari, Y. D. melanogaster argonaute1 and argonaute2 employ distinct mechanisms for translational repression. Mol. Cell 34, 58–67 (2009).
Beilharz, T.H. et al. microRNA-mediated messenger RNA deadenylation contributes to translational repression in mammalian cells. PLoS ONE 4, e6783 (2009).
Ricci, E.P. et al. Activation of a microRNA response in trans reveals a new role for poly(A) in translational repression. Nucleic Acids Res. 39, 5215–5231 (2011).
Thangima Zannat, M., Bhattacharjee, R.B. & Bag, J. Depletion of cellular poly (A) binding protein prevents protein synthesis and leads to apoptosis in HeLa cells. Biochem. Biophys. Res. Commun. 408, 375–381 (2011).
Blagden, S.P. et al. D. melanogaster Larp associates with poly(A)-binding protein and is required for male fertility and syncytial embryo development. Dev. Biol. 334, 186–197 (2009).
Kahvejian, A., Svitkin, Y.V., Sukarieh, R., M'Boutchou, M.N. & Sonenberg, N. Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms. Genes Dev. 19, 104–113 (2005).
Thermann, R. & Hentze, M.W. D. melanogaster miR2 induces pseudo-polysomes and inhibits translation initiation. Nature 447, 875–878 (2007).
Till, S. et al. A conserved motif in Argonaute-interacting proteins mediates functional interactions through the Argonaute PIWI domain. Nat. Struct. Mol. Biol. 14, 897–903 (2007).
Svitkin, Y.V. & Sonenberg, N. An efficient system for cap- and poly(A)-dependent translation in vitro. Methods Mol. Biol. 257, 155–170 (2004).
Moretti, F., Thermann, R. & Hentze, M.W. Mechanism of translational regulation by miR-2 from sites in the 5′ untranslated region or the open reading frame. RNA 16, 2493–2502 (2010).
Bartel, D.P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Eulalio, A. et al. The RRM domain in GW182 proteins contributes to miRNA-mediated gene silencing. Nucleic Acids Res. 37, 2974–2983 (2009).
Chekulaeva, M., Filipowicz, W. & Parker, R. Multiple independent domains of dGW182 function in miRNA-mediated repression in D. melanogaster. RNA 15, 794–803 (2009).
Tan, R. & Frankel, A.D. Structural variety of arginine-rich RNA-binding peptides. Proc. Natl. Acad. Sci. USA 92, 5282–5286 (1995).
Tomari, Y., Du, T. & Zamore, P.D. Sorting of D. melanogaster small silencing RNAs. Cell 130, 299–308 (2007).
Stark, A., Brennecke, J., Russel, R.B. & Cohen, S.M. Identification of D. melanogaster microRNA targets. PLoS Biol. 1, e60 (2003).
Chekulaeva, M. et al. miRNA repression involves GW182-mediated recruitment of CCR4-NOT through conserved W-containing motifs. Nat. Struct. Mol. Biol. 18, 1218–1226 (2011).
Simón, E. & Seraphin, B. A specific role for the C-terminal region of the poly(A)-binding protein in mRNA decay. Nucleic Acids Res. 35, 6017–6028 (2007).
Tucker, M., Staples, R.R., Valencia-Sanchez, M.A., Muhlrad, D. & Parker, R. Ccr4p is the catalytic subunit of a Ccr4p/Pop2p/Notp mRNA deadenylase complex in Saccharomyces cerevisiae. EMBO J. 21, 1427–1436 (2002).
Fabian, M.R. et al. miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4-NOT. Nat. Struct. Mol. Biol. 18, 1211–1217 (2011).
Kim, H.H. et al. HuR recruits let-7/RISC to repress c-Myc expression. Genes Dev. 23, 1743–1748 (2009).
Grskovic, M., Hentze, M.W. & Gebauer, F. A co-repressor assembly nucleated by Sex-lethal in the 3′UTR mediates translational control of D. melanogaster msl-2 mRNA. EMBO J. 22, 5571–5581 (2003).
Thoma, C., Fraterman, S., Gentzel, M., Wilm, M. & Hentze, M.W. Translation initiation by the c-myc mRNA internal ribosome entry sequence and the poly(A) tail. RNA 14, 1579–1589 (2008).
Czaplinski, K. et al. Identification of 40LoVe, a Xenopus hnRNP D family protein involved in localizing a TGF-β-related mRNA during oogenesis. Dev. Cell 8, 505–515 (2005).
Acknowledgements
We thank E. Izaurralde (Max Planck Institute for Developmental Biology, Tübingen, Germany) for GW182-SD-pAC5.1 and MBP-DmPABPC1 plasmids and for eIF4G antibody; C. Strein (EMBL, Heidelberg, Germany) for cloning and expression of Gst-λN-tagged GW182-SD; E. Izaurralde, L. Zekri and E. Huntzinger (Tübingen) for sharing unpublished results and their comments on this work; and all members of the Hentze laboratory, especially K. Duncan and J. Medenbach, for advice and discussions. This work was funded by a grant (FOR855 He 1442/13-2) from the Deutsche Forschungsgemeinschaft to M.W.H. C.K. is supported by an International Incoming Marie Curie fellowship (European Framework 7th, no. 253415).
Author information
Authors and Affiliations
Contributions
F.M., C.K., A.Z.-S. and M.W.H. designed experiments and analyzed results. F.M., C.K. and A.Z.-S. carried out experiments. F.M., C.K. and M.W.H. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 4353 kb)
Rights and permissions
About this article
Cite this article
Moretti, F., Kaiser, C., Zdanowicz-Specht, A. et al. PABP and the poly(A) tail augment microRNA repression by facilitated miRISC binding. Nat Struct Mol Biol 19, 603–608 (2012). https://doi.org/10.1038/nsmb.2309
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2309
This article is cited by
-
LncRNA PRBC induces autophagy to promote breast cancer progression through modulating PABPC1-mediated mRNA stabilization
Oncogene (2024)
-
MicroRNAs from plants to animals, do they define a new messenger for communication?
Nutrition & Metabolism (2018)
-
Deconvolution of seed and RNA-binding protein crosstalk in RNAi-based functional genomics
Nature Genetics (2018)
-
The 3′ end of the story: deciphering combinatorial interactions that control mRNA fate
Genome Biology (2017)
-
The influence of microRNAs and poly(A) tail length on endogenous mRNA–protein complexes
Genome Biology (2017)