Abstract
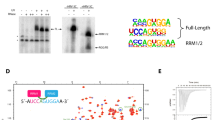
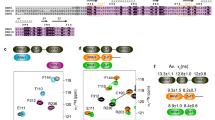
Tra2-β1 is a unique splicing factor as its single RNA recognition motif (RRM) is located between two RS (arginine-serine) domains. To understand how this protein recognizes its RNA target, we solved the structure of Tra2-β1 RRM in complex with RNA. The central 5′-AGAA-3′ motif is specifically recognized by residues from the β-sheet of the RRM and by residues from both extremities flanking the RRM. The structure suggests that RNA binding by Tra2-β1 induces positioning of the two RS domains relative to one another. By testing the effect of Tra2-β1 and RNA mutations on the splicing of SMN2 exon 7, we validated the importance of the RNA-protein contacts observed in the structure for the function of Tra2-β1 and determined the functional sequence of Tra2-β1 in SMN2 exon 7. Finally, we propose a model for the assembly of multiple RNA binding proteins on this exon.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
25 March 2011
In the version of this article initially published online, in Figure 2c, the label "A1" in the right-hand panel was displaced to the left, and in the Results, "Tra2-β1" was incorrectly written as "Tra2-b1" in the subheading "Tra2-β1 binding to SMN might change position of RS domains." The errors have been corrected for all versions of this article.
References
Novoyatleva, T. et al. Protein phosphatase 1 binds to the RNA recognition motif of several splicing factors and regulates alternative pre-mRNA processing. Hum. Mol. Genet. 17, 52–70 (2008).
Dauwalder, B., Amaya-Manzanares, F. & Mattox, W. A human homologue of the Drosophila sex determination factor transformer-2 has conserved splicing regulatory functions. Proc. Natl. Acad. Sci. USA 93, 9004–9009 (1996).
Nayler, O., Cap, C. & Stamm, S. Human transformer-2-beta gene (SFRS10): complete nucleotide sequence, chromosomal localization, and generation of a tissue-specific isoform. Genomics 53, 191–202 (1998).
Daoud, R., Da Penha Berzaghi, M., Siedler, F., Hubener, M. & Stamm, S. Activity-dependent regulation of alternative splicing patterns in the rat brain. Eur. J. Neurosci. 11, 788–802 (1999).
Beil, B., Screaton, G. & Stamm, S. Molecular cloning of htra2-beta-1 and htra2-beta-2, two human homologs of tra-2 generated by alternative splicing. DNA Cell Biol. 16, 679–690 (1997).
Hertel, K.J. & Graveley, B.R. RS domains contact the pre-mRNA throughout spliceosome assembly. Trends Biochem. Sci. 30, 115–118 (2005).
Shen, H. & Green, M.R. A pathway of sequential arginine-serine-rich domain-splicing signal interactions during mammalian spliceosome assembly. Mol. Cell 16, 363–373 (2004).
Shen, H. & Green, M.R. RS domains contact splicing signals and promote splicing by a common mechanism in yeast through humans. Genes Dev. 20, 1755–1765 (2006).
Shen, H. & Green, M.R. RS domain-splicing signal interactions in splicing of U12-type and U2-type introns. Nat. Struct. Mol. Biol. 14, 597–603 (2007).
Stoilov, P., Daoud, R., Nayler, O. & Stamm, S. Human tra2-beta1 autoregulates its protein concentration by influencing alternative splicing of its pre-mRNA. Hum. Mol. Genet. 13, 509–524 (2004).
Tacke, R., Tohyama, M., Ogawa, S. & Manley, J.L. Human Tra2 proteins are sequence-specific activators of pre-mRNA splicing. Cell 93, 139–148 (1998).
Tacke, R. & Manley, J.L. The human splicing factors ASF/SF2 and SC35 possess distinct, functionally significant RNA binding specificities. EMBO J. 14, 3540–3551 (1995).
Bourgeois, C.F., Lejeune, F. & Stevenin, J. Broad specificity of SR (serine/arginine) proteins in the regulation of alternative splicing of pre-messenger RNA. Prog. Nucleic Acid Res. Mol. Biol. 78, 37–88 (2004).
Burghes, A.H. & Beattie, C.E. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat. Rev. Neurosci. 10, 597–609 (2009).
Cartegni, L. & Krainer, A.R. Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat. Genet. 30, 377–384 (2002).
Kashima, T. & Manley, J.L. A negative element in SMN2 exon 7 inhibits splicing in spinal muscular atrophy. Nat. Genet. 34, 460–463 (2003).
Hofmann, Y., Lorson, C.L., Stamm, S., Androphy, E.J. & Wirth, B. Htra2-beta 1 stimulates an exonic splicing enhancer and can restore full-length SMN expression to survival motor neuron 2 (SMN2). Proc. Natl. Acad. Sci. USA 97, 9618–9623 (2000).
Hofmann, Y. & Wirth, B. hnRNP-G promotes exon 7 inclusion of survival motor neuron (SMN) via direct interaction with Htra2-beta1. Hum. Mol. Genet. 11, 2037–2049 (2002).
Young, P.J. et al. SRp30c-dependent stimulation of survival motor neuron (SMN) exon 7 inclusion is facilitated by a direct interaction with hTra2 beta 1. Hum. Mol. Genet. 11, 577–587 (2002).
Singh, N.N., Androphy, E.J. & Singh, R.N. In vivo selection reveals combinatorial controls that define a critical exon in the spinal muscular atrophy genes. RNA 10, 1291–1305 (2004).
Miyajima, H., Miyaso, H., Okumura, M., Kurisu, J. & Imaizumi, K. Identification of a cis-acting element for the regulation of SMN exon 7 splicing. J. Biol. Chem. 277, 23271–23277 (2002).
Singh, N.N., Shishimorova, M., Cao, L.C., Gangwani, L. & Singh, R.N. A short antisense oligonucleotide masking a unique intronic motif prevents skipping of a critical exon in spinal muscular atrophy. RNA Biol. 6, 341–350 (2009).
Lefebvre, S. et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80, 155–165 (1995).
Horne, C. & Young, P.J. Is RNA manipulation a viable therapy for spinal muscular atrophy? J. Neurol. Sci. 287, 27–31 (2009).
Golovanov, A.P., Hautbergue, G.M., Wilson, S.A. & Lian, L.Y. A simple method for improving protein solubility and long-term stability. J. Am. Chem. Soc. 126, 8933–8939 (2004).
Auweter, S.D., Oberstrass, F.C. & Allain, F.H. Sequence-specific binding of single-stranded RNA: is there a code for recognition? Nucleic Acids Res. 34, 4943–4959 (2006).
Heinrich, B. et al. Heterogeneous nuclear ribonucleoprotein G regulates splice site selection by binding to CC(A/C)-rich regions in pre-mRNA. J. Biol. Chem. 284, 14303–14315 (2009).
Simard, M.J. & Chabot, B. SRp30c is a repressor of 3′ splice site utilization. Mol. Cell. Biol. 22, 4001–4010 (2002).
Shen, H., Kan, J.L. & Green, M.R. Arginine-serine-rich domains bound at splicing enhancers contact the branchpoint to promote prespliceosome assembly. Mol. Cell 13, 367–376 (2004).
Skrisovska, L. et al. The testis-specific human protein RBMY recognizes RNA through a novel mode of interaction. EMBO Rep. 8, 372–379 (2007).
Cléry, A., Blatter, M. & Allain, F.H. RNA recognition motifs: boring? Not quite. Curr. Opin. Struct. Biol. 18, 290–298 (2008).
Maris, C., Dominguez, C. & Allain, F.H. The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J. 272, 2118–2131 (2005).
Deo, R.C., Bonanno, J.B., Sonenberg, N. & Burley, S.K. Recognition of polyadenylate RNA by the poly(A)-binding protein. Cell 98, 835–845 (1999).
Hargous, Y. et al. Molecular basis of RNA recognition and TAP binding by the SR proteins SRp20 and 9G8. EMBO J. 25, 5126–5137 (2006).
Mazza, C., Segref, A., Mattaj, I.W. & Cusack, S. Large-scale induced fit recognition of an m(7)GpppG cap analogue by the human nuclear cap-binding complex. EMBO J. 21, 5548–5557 (2002).
Tsuda, K. et al. Structural basis for the sequence-specific RNA-recognition mechanism of human CUG-BP1 RRM3. Nucleic Acids Res. 37, 5151–5166 (2009).
Ding, J. et al. Crystal structure of the two-RRM domain of hnRNP A1 (UP1) complexed with single-stranded telomeric DNA. Genes Dev. 13, 1102–1115 (1999).
Oberstrass, F.C. et al. Structure of PTB bound to RNA: specific binding and implications for splicing regulation. Science 309, 2054–2057 (2005).
Vitali, F. et al. Structure of the two most C-terminal RNA recognition motifs of PTB using segmental isotope labeling. EMBO J. 25, 150–162 (2006).
Irimura, S. et al. HnRNP C1/C2 may regulate exon 7 splicing in the spinal muscular atrophy gene SMN1. Kobe J. Med. Sci. 54, E227–E236 (2009).
Lynch, K.W. & Maniatis, T. Synergistic interactions between two distinct elements of a regulated splicing enhancer. Genes Dev. 9, 284–293 (1995).
Prior, T.W. et al. A positive modifier of spinal muscular atrophy in the SMN2 gene. Am. J. Hum. Genet. 85, 408–413 (2009).
Vezain, M. et al. A rare SMN2 variant in a previously unrecognized composite splicing regulatory element induces exon 7 inclusion and reduces the clinical severity of spinal muscular atrophy. Hum. Mutat. 31, E1110–E1125 (2010).
Lorson, C.L., Hahnen, E., Androphy, E.J. & Wirth, B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 96, 6307–6311 (1999).
Monani, U.R. et al. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 8, 1177–1183 (1999).
D'Souza, I. et al. Missense and silent tau gene mutations cause frontotemporal dementia with parkinsonism-chromosome 17 type, by affecting multiple alternative RNA splicing regulatory elements. Proc. Natl. Acad. Sci. USA 96, 5598–5603 (1999).
Rizzu, P. et al. High prevalence of mutations in the microtubule-associated protein tau in a population study of frontotemporal dementia in the Netherlands. Am. J. Hum. Genet. 64, 414–421 (1999).
Jiang, Z. et al. Mutations in tau gene exon 10 associated with FTDP-17 alter the activity of an exonic splicing enhancer to interact with Tra2 beta. J. Biol. Chem. 278, 18997–19007 (2003).
Koradi, R., Billeter, M. & Wuthrich, K. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graph. 14, 51–55 (1996).
Sattler, M., Schleucher, J. & Griesinger, C. Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog. NMR Spectrosc. 34, 93–158 (1999).
Peterson, R.D., Theimer, C.A., Wu, H. & Feigon, J. New applications of 2D filtered/edited NOESY for assignment and structure elucidation of RNA and RNA-protein complexes. J. Biomol. NMR 28, 59–67 (2004).
Wenter, P., Reymond, L., Auweter, S.D., Allain, F.H. & Pitsch, S. Short, synthetic and selectively 13C-labeled RNA sequences for the NMR structure determination of protein-RNA complexes. Nucleic Acids Res. 34, e79 (2006).
Lee, W., Revington, M.J., Arrowsmith, C. & Kay, L.E. A pulsed field gradient isotope-filtered 3D 13C HMQC-NOESY experiment for extracting intermolecular NOE contacts in molecular complexes. FEBS Lett. 350, 87–90 (1994).
Herrmann, T., Guntert, P. & Wuthrich, K. Protein NMR structure determination with automated NOE-identification in the NOESY spectra using the new software ATNOS. J. Biomol. NMR 24, 171–189 (2002).
Herrmann, T., Guntert, P. & Wuthrich, K. Protein NMR structure determination with automated NOE assignment using the new software CANDID and the torsion angle dynamics algorithm DYANA. J. Mol. Biol. 319, 209–227 (2002).
Case, D.A. et al. The Amber biomolecular simulation programs. J. Comput. Chem. 26, 1668–1688 (2005).
Auweter, S.D. et al. Molecular basis of RNA recognition by the human alternative splicing factor Fox-1. EMBO J. 25, 163–173 (2006).
Laskowski, R.A., Rullmannn, J.A., MacArthur, M.W., Kaptein, R. & Thornton, J.M. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 8, 477–486 (1996).
Hartmann, A.M., Nayler, O., Schwaiger, F.W., Obermeier, A. & Stamm, S. The interaction and colocalization of Sam68 with the splicing-associated factor YT521-B in nuclear dots is regulated by the Src family kinase p59(fyn). Mol. Biol. Cell 10, 3909–3926 (1999).
Acknowledgements
We thank J. Stevenin and D. Watt for reading the manuscript; J. Hall and M. Zimmermann for labeled RNA; the SNF-NCCR Structural Biology and EURASNET for financial support to F.H.-T.A.; the Muscular Dystrophy Association for support of S.S. and the European Molecular Biology Organization for a postdoctoral fellowship to A.C.
Author information
Authors and Affiliations
Contributions
F.H.-T.A. and S.S. designed the project; A.C. prepared protein and RNA samples for structural studies; A.C., C.D. and F.H.-T.A. analyzed NMR data; A.C. and C.D. did structure calculations; S.J., N.B. and A.C. did in vivo splicing assays; A.C. and C.D. did ITC measurements; N.B. conducted the coimmunoprecipitation experiments; all authors discussed the results and wrote and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Methods (PDF 2214 kb)
Rights and permissions
About this article
Cite this article
Cléry, A., Jayne, S., Benderska, N. et al. Molecular basis of purine-rich RNA recognition by the human SR-like protein Tra2-β1. Nat Struct Mol Biol 18, 443–450 (2011). https://doi.org/10.1038/nsmb.2001
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2001
This article is cited by
-
Autonomous transposons tune their sequences to ensure somatic suppression
Nature (2024)
-
Heat increases full-length SMN splicing: promise for splice-augmenting therapies for SMA
Human Genetics (2022)
-
Structure of SRSF1 RRM1 bound to RNA reveals an unexpected bimodal mode of interaction and explains its involvement in SMN1 exon7 splicing
Nature Communications (2021)
-
Transcriptional control of CBX5 by the RNA-binding proteins RBMX and RBMXL1 maintains chromatin state in myeloid leukemia
Nature Cancer (2021)
-
Specific inhibition of splicing factor activity by decoy RNA oligonucleotides
Nature Communications (2019)