Abstract
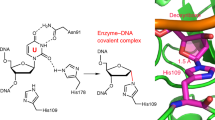
Prokaryotic tRNA guanine transglycosylase (TGT) catalyzes replacement of guanine (G) by 7-aminomethyl-7-deazaguanine (PreQ1) at the wobble position of four specific tRNAs. Addition of 9-deazaguanine (9dzG) to a reaction mixture of Zymomonas mobilis TGT and an RNA substrate allowed us to trap, purify and crystallize a chemically competent covalent intermediate of the TGT-catalyzed reaction. The crystal structure of the TGT–RNA–9dzG ternary complex at a resolution of 2.9 Å reveals, unexpectedly, that RNA is tethered to TGT through the side chain of Asp280. Thus, Asp280, instead of the previously proposed Asp102, acts as the nucleophile for the reaction. The RNA substrate adopts an unusual conformation, with four out of seven nucleotides in the loop region flipped out. Interactions between TGT and RNA revealed by the structure provide the molecular basis of the RNA substrate requirements by TGT. Furthermore, reaction of PreQ1 with the crystallized covalent intermediate provides insight into the necessary structural changes required for the TGT-catalyzed reaction to occur.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Limbach, P.A., Crain, P.F. & McCloskey, J.A. Summary: the modified nucleosides of RNA. Nucleic Acids Res. 22, 2183–2196 (1994).
Urbonavicius, J., Qian, Q., Durand, J.M., Hagervall, T.G. & Bjork, G.R. Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J. 20, 4863–4873 (2001).
Yarian, C. et al. Accurate translation of the genetic code depends on tRNA modified nucleosides. J. Biol. Chem. 277, 16391–16395 (2002).
Yasukawa, T., Suzuki, T., Ishii, N., Ohta, S. & Watanabe, K. Wobble modification defect in tRNA disturbs codon-anticodon interaction in a mitochondrial disease. EMBO J. 20, 4794–4802 (2001).
Yasukawa, T. et al. Defect in modification at the anticodon wobble nucleotide of mitochondrial tRNALys with the MERRF encephalomyopathy pathogenic mutation. FEBS Lett. 467, 175–178 (2000).
Okada, N. & Nishimura, S. Isolation and characterization of a guanine insertion enzyme, a specific tRNA transglycosylase, from Escherichia coli. J. Biol. Chem. 254, 3061–3066 (1979).
Okada, N. et al. Novel mechanism of post-transcriptional modification of tRNA. Insertion of bases of Q precursors into tRNA by a specific tRNA transglycosylase reaction. J. Biol. Chem. 254, 3067–3073 (1979).
Slany, R.K., Bosl, M., Crain, P.F. & Kersten, H. A new function of S-adenosylmethionine: the ribosyl moiety of AdoMet is the precursor of the cyclopentenediol moiety of the tRNA wobble base queuine. Biochemistry 32, 7811–7817 (1993).
Frey, B., McCloskey, J., Kersten, W. & Kersten, H. New function of vitamin B12: cobamide-dependent reduction of epoxyqueuosine to queuosine in tRNAs of Escherichia coli and Salmonella typhimurium. J. Bacteriol. 170, 2078–2082 (1988).
Katze, J.R., Gunduz, U., Smith, D.L., Cheng, C.S. & McCloskey, J.A. Evidence that the nucleic acid base queuine is incorporated intact into tRNA by animal cells. Biochemistry 23, 1171–1176 (1984).
Curnow, A.W., Kung, F.L., Koch, K.A. & Garcia, G.A. tRNA-guanine transglycosylase from Escherichia coli: gross tRNA structural requirements for recognition. Biochemistry 32, 5239–5246 (1993).
Nakanishi, S. et al. A UGU sequence in the anticodon loop is a minimum requirement for recognition by Escherichia coli tRNA-guanine transglycosylase. J. Biol. Chem. 269, 32221–32225 (1994).
Curnow, A.W. & Garcia, G.A. tRNA-guanine transglycosylase from Escherichia coli. Minimal tRNA structure and sequence requirements for recognition. J. Biol. Chem. 270, 17264–17267 (1995).
Romier, C., Reuter, K., Suck, D. & Ficner, R. Crystal structure of tRNA-guanine transglycosylase: RNA modification by base exchange. EMBO J. 15, 2850–2857 (1996).
Romier, C., Reuter, K., Suck, D. & Ficner, R. Mutagenesis and crystallographic studies of Zymomonas mobilis tRNA-guanine transglycosylase reveal aspartate 102 as the active site nucleophile. Biochemistry 35, 15734–15739 (1996).
Kung, F.L., Nonekowski, S. & Garcia, G.A. tRNA-guanine transglycosylase from Escherichia coli: recognition of noncognate-cognate chimeric tRNA and discovery of a novel recognition site within the TpsiC arm of tRNAPhe. RNA 6, 233–244 (2000).
Banner, D.W., Bloomer, A., Petsko, G.A., Phillips, D.C. & Wilson, I.A. Atomic coordinates for triose phosphate isomerase from chicken muscle. Biochem. Biophys. Res. Commun. 72, 146–155 (1976).
Shi, H. & Moore, P.B. The crystal structure of yeast phenylalanine tRNA at 1.93 Å resolution: a classic structure revisited. RNA 6, 1091–1105 (2000).
Westhof, E., Dumas, P. & Moras, D. Restrained refinement of two crystalline forms of yeast aspartic acid and phenylalanine transfer RNA crystals. Acta Crystallogr. A 44, 112–123 (1988).
Benas, P. et al. The crystal structure of HIV reverse-transcription primer tRNA(Lys,3) shows a canonical anticodon loop. RNA 6, 1347–1355 (2000).
Basavappa, R. & Sigler, P.B. The 3 Å crystal structure of yeast initiator tRNA: functional implications in initiator/elongator discrimination. EMBO J. 10, 3105–3111 (1991).
Ruff, M. et al. Class II aminoacyl transfer RNA synthetases: crystal structure of yeast aspartyl-tRNA synthetase complexed with tRNAAsp. Science 252, 1682–1689 (1991).
Goldgur, Y. et al. The crystal structure of phenylalanyl-tRNA synthetase from Thermus thermophilus complexed with cognate tRNAPhe. Structure 5, 59–68 (1997).
Rould, M.A., Perona, J.J. & Steitz, T.A. Structural basis of anticodon loop recognition by glutaminyl-tRNA synthetase. Nature 352, 213–218 (1991).
Ishitani, R. et al. Crystal structure of archaeosine tRNA-guanine transglycosylase. J. Mol. Biol. 318, 665–677 (2002).
Marck, C. & Grosjean, H. tRNomics: analysis of tRNA genes from 50 genomes of Eukarya, Archaea, and Bacteria reveals anticodon-sparing strategies and domain-specific features. RNA 8, 1189–1232 (2002).
Auffinger, P. & Westhof, E. An extended structural signature for the tRNA anticodon loop. RNA 7, 334–341 (2001).
Hoops, G.C., Park, J., Garcia, G.A. & Townsend, L.B. Determination of acidic ionization constants of certain 5-substituted 2-aminopyrrolo[2,3-d]pyrimidin-4-ones. J. Heterocyclic Chem. 33, 767–781 (1996).
Slupphaug, G. et al. A nucleotide-flipping mechanism from the structure of human uracil-DNA glycosylase bound to DNA. Nature 384, 87–92 (1996).
Lau, A.Y., Scharer, O.D., Samson, L., Verdine, G.L. & Ellenberger, T. Crystal structure of a human alkylbase-DNA repair enzyme complexed to DNA: mechanisms for nucleotide flipping and base excision. Cell 95, 249–258 (1998).
Bruner, S.D., Norman, D.P. & Verdine, G.L. Structural basis for recognition and repair of the endogenous mutagen 8-oxoguanine in DNA. Nature 403, 859–866 (2000).
Romier, C., Ficner, R., Reuter, K. & Suck, D. Purification, crystallization, and preliminary X-ray diffraction studies of tRNA-guanine transglycosylase from Zymomonas mobilis. Proteins 24, 516–519 (1996).
Tayler, E.C. & Young, W.B. Pyrrolo[3,2-d]pyrimidine folate analogues: “inverted” analogues of the cytotoxic agent LY231514. J. Org. Chem. 60, 7947–7952 (1995).
Akimoto, H., Imamiya, E., Hitaka, T. & Nomura, H. Synthesis of queuine, the base of naturally occurring hypermodified nucleoside (queuosine) and its analogues. J. Chem. Perkin Trans. I, 1637–1644 (1988).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 277, 307–326 (1997).
Navaza, J. AMoRe: an automated package for molecular replacement. Acta Crystallogr. A 50, 157–163 (1994).
Jones, T.A., Zou, J.-Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Brünger, A.T. et al. Crystallography & NMR System: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Carson, M. Ribbon models of macromolecules. J. Mol. Graph. 5, 103–106 (1987).
Read, R.J. Improved Fourier coefficients for maps using phases from partial structures with errors. Acta Crystallogr. A 42, 140–149 (1986).
Ishitani, R. et al. Alternative tertiary structure of tRNA for recognition by a posttranscriptional modification enzyme. Cell 113, 383–394 (2003).
Acknowledgements
We are grateful to T. Conway (University of Oklahoma) for a plasmid encoding the Z. mobilis TGT and to P. Tyler (Industrial Research Ltd., New Zealand) for providing the initial 9dzG. We thank the staffs of beamlines 14-BMC (K. Brister, T. Teng and R. Pahl) and 19-ID (S. Ginell, A. Joachimiak and Y. Kim) at APS for their support during data collections; Y. Elias, K. Phannach and other members of the Huang research group; and D. Kranz, R. Switzer and J. Gerlt for many helpful discussions and critical reading of the manuscript. The work was supported by start-up funds from the University of Illinois and a grant from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Xie, W., Liu, X. & Huang, R. Chemical trapping and crystal structure of a catalytic tRNA guanine transglycosylase covalent intermediate. Nat Struct Mol Biol 10, 781–788 (2003). https://doi.org/10.1038/nsb976
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb976
This article is cited by
-
Individually double minimum-distance definition of protein–RNA binding residues and application to structure-based prediction
Journal of Computer-Aided Molecular Design (2018)
-
Structures of human ADAR2 bound to dsRNA reveal base-flipping mechanism and basis for site selectivity
Nature Structural & Molecular Biology (2016)
-
Structural basis for translational fidelity ensured by transfer RNA lysidine synthetase
Nature (2009)
-
Tertiary structure checkpoint at anticodon loop modification in tRNA functional maturation
Nature Structural & Molecular Biology (2009)
-
Crystal structure of Staphylococcus aureus tRNA adenosine deaminase TadA in complex with RNA
Nature Structural & Molecular Biology (2006)