Abstract
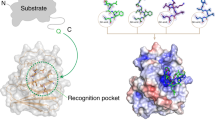
The structural proteins of HIV and Ebola display PTAP peptide motifs (termed 'late domains') that recruit the human protein Tsg101 to facilitate virus budding. Here we present the solution structure of the UEV (ubiquitin E2 variant) binding domain of Tsg101 in complex with a PTAP peptide that spans the late domain of HIV-1 p6Gag. The UEV domain of Tsg101 resembles E2 ubiquitin-conjugating enzymes, and the PTAP peptide binds in a bifurcated groove above the vestigial enzyme active site. Each PTAP residue makes important contacts, and the Ala 9-Pro 10 dipeptide binds in a deep pocket of the UEV domain that resembles the X-Pro binding pockets of SH3 and WW domains. The structure reveals the molecular basis of HIV PTAP late domain function and represents an attractive starting point for the design of novel inhibitors of virus budding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Freed, E.O. J. Virol. 76, 4679–4687 (2002).
Pornillos, O.P., Garrus, J.E. & Sundquist, W.I. Trends Cell Biol. in the press (2002).
Göttlinger, H.G. AIDS 15, S13–S20 (2001).
Göttlinger, H.G., Dorfman, T., Sodroski, J.G. & Haseltine, W.A. Proc. Natl. Acad. Sci. USA 88, 3195–3199 (1991).
Huang, M., Orenstein, J.M., Martin, M.A. & Freed, E.O. J. Virol. 69, 6810–6818 (1995).
Demirov, D.G., Orenstein, J.M. & Freed, E.O. J. Virol. 76, 105–117 (2002).
VerPlank, L. et al. Proc. Natl. Acad. Sci. USA 98, 7724–7729 (2001).
Garrus, J.E. et al. Cell 107, 55–65 (2001).
Martin-Serrano, J., Zang, T. & Bieniasz, P.D. Nature Med. 7, 1313–1319 (2001).
Demirov, D.G., Ono, A., Orenstein, J.M. & Freed, E.O. Proc. Natl. Acad. Sci. USA 99, 955–960 (2002).
Strack, B., Calistri, A., Accola, M.A., Palu, G. & Göttlinger, H.G. Proc. Natl. Acad. Sci. USA 97, 13063–13068 (2000).
Schubert, U. et al. Proc. Natl. Acad. Sci. USA 97, 13057–13062 (2000).
Patnaik, A., Chau, V. & Wills, J.W. Proc. Natl. Acad. Sci. USA 97, 13069–13074 (2000).
Vogt, V.M. Proc. Natl. Acad. Sci. USA 97, 12945–12947 (2000).
Harty, R.N. et al. J. Virol. 75, 10623–10629 (2001).
Harty, R.N., Brown, M.E., Wang, G., Huibregtse, J. & Hayes, F.P. Proc. Natl. Acad. Sci. USA 97, 13871–13876 (2000).
Hicke, L. Cell 106, 527–530 (2001).
Strack, B., Calistri, A. & Göttlinger, H.G. J. Virol. 76, 5472–5479 (2002).
Ott, D.E. et al. J. Virol. 72, 2962–2968 (1998).
Ott, D.E., Coren, L.V., Chertova, E.N., Gagliardi, T.D. & Schubert, U. Virology 278, 111–121 (2000).
Lemmon, S.K. & Traub, L.M. Curr. Opin. Cell Biol. 12, 457–466 (2000).
Katzmann, D.J., Babst, M. & Emr, S.D. Cell 106, 145–155 (2001).
Bishop, N., Horman, A. & Woodman, P. J. Cell Biol. 157, 91–102 (2002).
Dupre, S., Volland, C. & Haguenauer-Tsapis, R. Curr. Biol. 11, R932–R934 (2001).
Pornillos, O. et al. EMBO J. 21, 2397–2406 (2002).
VanDemark, A.P., Hofmann, R.M., Tsui, C., Pickart, C.M. & Wolberger, C. Cell 105, 711–720 (2001).
Moraes, T.F. et al. Nature Struct. Biol. 8, 669–673 (2001).
Koonin, E.V. & Abagyan, R.A. Nature Genet. 16, 330–331 (1997).
Ponting, C.P., Cai, Y.D. & Bork, P. J. Mol. Med. 75, 467–469 (1997).
Sancho, E. et al. Mol. Cell. Biol. 18, 576–589 (1998).
Pickart, C.M. Annu. Rev. Biochem. 70, 503–533 (2001).
Zarrinpar, A. & Lim, W.A. Nature Struct. Biol. 7, 611–613 (2000).
Kay, B.K., Williamson, M.P. & Sudol, M. FASEB J. 14, 231–241 (2000).
Huang, X. et al. Nature Struct. Biol. 7, 634–638 (2000).
Wittekind, M. et al. J. Mol. Biol. 267, 933–952 (1997).
Kuiken, C. et al. HIV Sequence Compendium (Los Alamos National Laboratory, Los Alamos; 2000).
Piper, R.C., Cooper, A.A., Yang, H. & Stevens, T.H. J. Cell Biol. 131, 603–617 (1995).
Raiborg, C., Bache, K.G., Mehlum, A. & Stenmark, H. Biochem. Soc. Trans. 29, 472–475 (2001).
Polo, S. et al. Nature 416, 451–455 (2002).
Pickart, C.M. Mol. Cell 8, 499–504 (2001).
Nguyen, J.T., Turck, C.W., Cohen, F.E., Zuckermann, R.N. & Lim, W.A. Science 282, 2088–2092 (1998).
Nguyen, J.T. et al. Chem. Biol. 7, 463–473 (2000).
Dadlez, M. & Kim, P.S. Biochemistry 35, 16153–16164 (1996).
Ferentz, A.E. & Wagner, G. Q. Rev. Biophys. 33, 29–65 (2000).
Ogura, K., Terasawa, H. & Inagaki, F. J. Magn. Reson. B 112, 63–68 (1996).
Ikura, M. & Bax, A. J. Am. Chem. Soc. 114, 2433–2440 (1992).
Zwahlen, C. et al. J. Am. Chem. Soc. 119, 6711–6721 (1997).
Güntert, P., Mumenthaler, C. & Wüthrich, K. J. Mol. Biol. 273, 283–298 (1997).
Brünger, A.T. et al. Acta Crystallogr. D 54, 905–921 (1998).
Laskowski, R.A., Rullmann, J.A., MacArthur, M.W., Kaptein, R. & Thornton, J.M. J. Biomol. NMR 8, 477–486 (1996).
Koradi, R., Billeter, M. & Wüthrich, K. J. Mol. Graph. 14, 51–55 (1996).
Kraulis, P.J. J. Appl. Crystallogr. 24, 946–950 (1991).
Wallace, A.C., Laskowski, R.A. & Thornton, J.M. Protein Eng. 8, 127–134 (1995).
Feng, G.H., Lih, C.J. & Cohen, S.N. Cancer Res. 60, 1736–1741 (2000).
Acknowledgements
We thank B. Schackmann and S. Endicott for peptide synthesis and purification, D. Edwards for technical NMR support and E. Ross for computer support. This work was supported by NIH funding to W.I.S. The Utah Biomolecular NMR Facility is supported by grants from the NIH and the NSF.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Pornillos, O., Alam, S., Davis, D. et al. Structure of the Tsg101 UEV domain in complex with the PTAP motif of the HIV-1 p6 protein. Nat Struct Mol Biol 9, 812–817 (2002). https://doi.org/10.1038/nsb856
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb856
This article is cited by
-
Backbone NMR resonance assignment of the apo human Tsg101-UEV domain
Biomolecular NMR Assignments (2023)
-
Citron kinase enhances ubiquitination of HIV-1 Gag protein and intracellular HIV-1 budding
Archives of Virology (2016)