Abstract
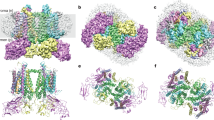
Here we present cryoelectron crystallographic analysis of an isolated dimeric oxygen-evolving complex of photosystem II (at a resolution of ~0.9 nm), revealing that the D1–D2 reaction center (RC) proteins are centrally located between the chlorophyll-binding proteins, CP43 and CP47. This conclusion supports the hypothesis that photosystems I and II have similar structural features and share a common evolutionary origin. Additional density connecting the two halves of the dimer, which was not observed in a recently described CP47–RC complex that did not include CP43, may be attributed to the small subunits that are involved in regulating secondary electron transfer, such as PsbH. These subunits are possibly also required for stabilization of the dimeric photosystem II complex. This complex, containing at least 29 transmembrane helices in its asymmetric unit, represents one of the largest membrane protein complexes studied at this resolution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Diner, B.A. & Babcock, G.T. In Oxygenic photosynthesis: the light reactions. (eds Ort, D.R. & Yocum, C.F.) 213–247 (Kluwer, Dordrecht, The Netherlands; 1996).
Michel, H. & Deisenhofer, J. Biochemistry 27, 1–7 (1988).
Rhee, K.-H., Morris, E.P., Barber, J. & Kühlbrandt, W. Nature 396, 283–286 (1998).
Bricker, T.M. Photosynth. Res. 24, 1–13 (1990).
Krauss, N. et al. Nature Struct. Biol. 3, 965–973 (1996).
Schubert, W.-D. et al. J. Mol. Biol. 272, 741–769 (1997).
Fromme, P. et al. Biochim. Biophys. Acta 1275, 76–83 (1996).
Schubert, W.-D. et al. J. Mol. Biol. 280, 297–314 (1998).
Barber, J., Nield, J., Morris, E.P. Zheleva, D. & Hankamer, B. Physiol. Plant. 100, 817–827 (1997).
Hankamer, B. et al. Eur. J. Biochem. 243, 422–429 (1997).
Cattucci, L. et al. In Photosynthesis: Mechanisms and Effects, vol. II (ed. Garab, G.) 973–976 (Kluwer, Dordrecht, The Netherlands; 1998).
Morris, E.P. Hankamer, B., Zheleva, D., Friso, G. & Barber, J. Structure 5, 837–849 (1997).
Marr, K.M., Mastronarde, D.N. & Lyon, M.K. J. Cell Biol. 132, 823–833 (1996).
Rhee, K.-H. et al. Nature 389, 522–526 (1997).
Boekema, E.J., Nield, J., Hankamer, B. & Barber, J. Eur J. Biochem. 252, 268–276 (1998).
Packham, N.K. FEBS Lett. 231, 284–290 (1988).
Mayes, S.R. et al. Biochemistry 32, 11454–1465 (1993).
Henderson, R. et al. J. Mol. Biol. 213, 899–929 (1990).
van Heel, M., Harauz, G., Orlova, E.V., Schmidt, R. & Schatz, M. J. Struct. Biol. 116, 17–24 (1996).
Henderson, R. et al. Ultramicroscopy 19, 147–178 (1986).
Laemmli, U.K. Nature 277, 680–685 (1970).
Moskalenko, A.A., Barbato, R. & Giacometti, G.M. FEBS Lett. 314, 271–274 (1992).
Barbato, R., Friso, G., Rigoni, F., Vecchia, F.D. & Giacometti, G.M. J. Cell Biol. 119, 325–335 (1992).
Acknowledgements
We thank the BBSRC for financial support, B. Gowen, S. Chen and H. Saibil for help with electron microscopy and sample preparation, M. van Heel for the use of a densitometer and computing facilities and J. Nield for preparing Fig. 1. We also thank W. Kühlbrandt and P. Fromme for valuable discussions on this work.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hankamer, B., Morris, E. & Barber, J. Revealing the structure of the oxygen-evolving core dimer of photosystem II by cryoelectron crystallography. Nat Struct Mol Biol 6, 560–564 (1999). https://doi.org/10.1038/9341
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/9341
This article is cited by
-
Cyclophilin anaCyp40 regulates photosystem assembly and phycobilisome association in a cyanobacterium
Nature Communications (2022)
-
Structural roles of lipid molecules in the assembly of plant PSII−LHCII supercomplex
Biophysics Reports (2018)
-
Structure of spinach photosystem II–LHCII supercomplex at 3.2 Å resolution
Nature (2016)
-
Effect of protein modification by malondialdehyde on the interaction between the oxygen-evolving complex 33 kDa protein and photosystem II core proteins
Planta (2010)
-
Substrate water binding and oxidation in photosystem II
Photosynthesis Research (2008)