Abstract
Erectile dysfunction (ED) is the most common sexual disorder reported by men to their health-care providers and the most investigated male sexual dysfunction. Currently, the treatment of ED focuses on 'symptomatic relief' of ED and, therefore, tends to provide temporary relief rather than providing a cure or reversing the cause. The identification of a large population of “difficult-to-treat” patients has triggered researchers to identify novel treatment approaches, which focus on cure and restoration of the underlying cause of ED. Regenerative medicine has developed extensively in the past few decades and preclinical trials have emphasized the benefit of growth factor therapy, gene transfer, stem cells and tissue engineering for the restoration of erectile function. Development of clinical trials involving immunomodulation in postprostatectomy ED patients and the use of maxi-K channels for gene therapy are illustrative of the advances in the field. However, the search for novel treatment targets and a wealth of preclinical studies represent a dynamic and continuing field of enquiry.
Key Points
-
Erectile dysfunction is the most commonly reported sexual disorder in men
-
A large subpopulation of men with ED are unresponsive to available oral pharmacotherapy (PDE5 inhibitors), owing to a loss of NO signalling and changes in penile tissue composition
-
Unresponsive patients might benefit from regenerative medicine options that restore tissue integrity and signalling in penis-projecting neurons, penile smooth muscle cells and endothelial cells
-
According to the degree of ED and tissue damage, regenerative medicine for ED includes tissue-protective pharmacotherapy, gene therapy, (stem) cell-based therapies or whole tissue replacement using bioengineered tissues
-
Aside from one clinical trial, these studies so far remain in the preclinical phase; however, current research is aiming to translate various regenerative treatments to clinical trials
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA 270, 83–90 (1993).
Uckert, S., Mayer, M. E., Stief, C. G. & Jonas, U. The future of the oral pharmacotherapy of male erectile dysfunction: things to come. Expert Opin. Emerg. Drugs 12, 219–228 (2007).
Derogatis, L. R. & Burnett, A. L. The epidemiology of sexual dysfunctions. J. Sex. Med. 5, 289–300 (2008).
Saigal, C. S., Wessells, H., Pace, J., Schonlau, M. & Wilt, T. J. Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch. Intern. Med. 166, 207–212 (2006).
Kim, N., Azadzoi, K. M., Goldstein, I. & Saenz de Tejada, I. A nitric oxide-like factor mediates nonadrenergic-noncholinergic neurogenic relaxation of penile corpus cavernosum smooth muscle. J. Clin. Invest. 88, 112–118 (1991).
Sáenz de Tejada, I. Molecular mechanisms for the regulation of penile smooth muscle contractility. Int. J. Impot. Res. 14 (Suppl. 1), S6–S10 (2002).
Lue, T. F. Erectile dysfunction. N. Engl. J. Med. 342, 1802–1813 (2000).
Albersen, M., Shindel, A. W., Mwamukonda, K. B. & Lue, T. F. The future is today: emerging drugs for the treatment of erectile dysfunction. Expert Opin. Emerg. Drugs 15, 467–480 (2010).
Hatzimouratidis, K. & Hatzichristou, D. Phosphodiesterase type 5 inhibitors: the day after. Eur. Urol. 51, 75–89 (2007).
Xie, D., Annex, B. H. & Donatucci, C. F. Growth factors for therapeutic angiogenesis in hypercholesterolemic erectile dysfunction. Asian J. Androl. 10, 23–27 (2008).
Lin, C.-S. & Lue, T. F. Growth factor therapy and neuronal nitric oxide synthase. Int. J. Impot. Res. 16 (Suppl. 1), S38–S39 (2004).
Bella, A. J., Lin, G., Cagiannos, I. & Lue, T. F. Emerging neuromodulatory molecules for the treatment of neurogenic erectile dysfunction caused by cavernous nerve injury. Asian J. Androl. 10, 54–59 (2008).
Bella, A. J. et al. Nerve growth factor modulation of the cavernous nerve response to injury. J. Sex. Med. 6 (Suppl. 3), 347–352 (2009).
Lin, G. et al. Neurotrophic effects of brain-derived neurotrophic factor and vascular endothelial growth factor in major pelvic ganglia of young and aged rats. BJU Int. 105, 114–120 (2010).
Bella, A. J. et al. Neurturin enhances the recovery of erectile function following bilateral cavernous nerve crush injury in the rat. J. Brachial Plex. Peripher. Nerve Inj. 2, 5 (2007).
Fandel, T. M. et al. Intracavernous growth differentiation factor-5 therapy enhances the recovery of erectile function in a rat model of cavernous nerve injury. J. Sex. Med. 5, 1866–1875 (2008).
Jung, G. W., Kwak, J. Y., Yoon, S., Yoon, J. H. & Lue, T. F. IGF-I and TGF-beta2 have a key role on regeneration of nitric oxide synthase (NOS)-containing nerves after cavernous neurotomy in rats. Int. J. Impot. Res. 11, 247–259 (1999).
Jung, G. W. et al. The role of growth factor on regeneration of nitric oxide synthase (NOS)-containing nerves after cavernous neurotomy in the rats. Int. J. Impot. Res. 11, 227–235 (1999).
Allaf, M. E., Hoke, A. & Burnett, A. L. Erythropoietin promotes the recovery of erectile function following cavernous nerve injury. J. Urol. 174, 2060–2064 (2005).
Albersen, M., Joniau, S., Claes, H. & Van Poppel, H. Preclinical evidence for the benefits of penile rehabilitation therapy following nerve-sparing radical prostatectomy. Adv. Urol. 594868 (2008).
Calenda, G. et al. Whole genome microarray of the major pelvic ganglion after cavernous nerve injury: new insights into molecular profile changes after nerve injury. BJU Int. 109, 1552–1564 (2012).
Bella, A. J. et al. Upregulation of penile brain-derived neurotrophic factor (BDNF) and activation of the JAK/STAT signalling pathway in the major pelvic ganglion of the rat after cavernous nerve transection. Eur. Urol. 52, 574–580 (2007).
Bond, C., Tang, Y. & Podlasek, C. A. Neural influences on sonic hedgehog and apoptosis in the rat penis. Biol. Reprod. 78, 947–956 (2008).
Bond, C. W. et al. Peptide amphiphile nanofiber delivery of sonic hedgehog protein to reduce smooth muscle apoptosis in the penis after cavernous nerve resection. J. Sex. Med. 8, 78–89 (2011).
Jin, H.-R. et al. Intracavernous delivery of synthetic angiopoietin-1 protein as a novel therapeutic strategy for erectile dysfunction in the type II diabetic db/db mouse. J. Sex. Med. 7, 3635–3646 (2010).
Jung, G. W., Spencer, E. M. & Lue, T. F. Growth hormone enhances regeneration of nitric oxide synthase-containing penile nerves after cavernous nerve neurotomy in rats. J. Urol. 160, 1899–1904 (1998).
Abdelbaky, T. M., Brock, G. B. & Huynh, H. Improvement of erectile function in diabetic rats by insulin: possible role of the insulin-like growth factor system. Endocrinology 139, 3143–3147 (1998).
Shirai, M. et al. Vascular endothelial growth factor restores erectile function through modulation of the insulin-like growth factor system and sex hormone receptors in diabetic rat. Biochem. Biophys. Res. Commun. 341, 755–762 (2006).
Pu, X.-Y. et al. Insulin-like growth factor-1 restores erectile function in aged rats: modulation the integrity of smooth muscle and nitric oxide-cyclic guanosine monophosphate signaling activity. J. Sex. Med. 5, 1345–1354 (2008).
Zhang, H.-Y., Jin, X.-B. & Lue, T. F. Three important components in the regeneration of the cavernous nerve: brain-derived neurotrophic factor, vascular endothelial growth factor and the JAK/STAT signaling pathway. Asian J. Androl. 13, 231–235 (2011).
Hsieh, P.-S. et al. The effect of vascular endothelial growth factor and brain-derived neurotrophic factor on cavernosal nerve regeneration in a nerve-crush rat model. BJU Int. 92, 470–475 (2003).
Bella, A. J. et al. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: part I. J. Sex. Med. 3, 815–820 (2006).
Lin, G., Bella, A. J., Lue, T. F. & Lin, C.-S. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: part 2. J. Sex. Med. 3, 821–829 (2006).
Albersen, M. et al. Pentoxifylline promotes recovery of erectile function in a rat model of postprostatectomy erectile dysfunction. Eur. Urol. 59, 286–296 (2011).
Yamashita, S. et al. Inhibition of interleukin-6 attenuates erectile dysfunction in a rat model of nerve-sparing radical prostatectomy. J. Sex. Med. 8, 1957–1964 (2011).
Lagoda, G., Sezen, S. F. & Burnett, A. L. FK506 and rapamycin neuroprotect erection and involve different immunophilins in a rat model of cavernous nerve injury. J. Sex. Med. 6, 1914–1923 (2009).
Hayashi, N. et al. The effect of FK1706 on erectile function following bilateral cavernous nerve crush injury in a rat model. J. Urol. 176, 824–829 (2006).
Valentine, H. et al. Neuroimmunophilin ligands protect cavernous nerves after crush injury in the rat: new experimental paradigms. Eur. Urol. 51, 1724–1731 (2007).
Sezen, S. F., Hoke, A., Burnett, A. L. & Snyder, S. H. Immunophilin ligand FK506 is neuroprotective for penile innervation. Nat. Med. 7, 1073–1074 (2001).
US National Library of Medicine. ClinicalTrials.gov [online], (2010).
US National Library of Medicine. ClinicalTrials.gov [online], (2008).
Burnett, A. L. Erectile dysfunction management for the future. J. Androl. 30, 391–396 (2009).
Hatzimouratidis, K. Editorial comment on: Smooth-muscle-specific gene transfer with the human maxi-K channel improves erectile function and enhances sexual behavior in atherosclerotic cynomolgus monkeys. Eur. Urol. 56, 1066 (2009).
Garbán, H. et al. Cloning of rat and human inducible penile nitric oxide synthase. Application for gene therapy of erectile dysfunction. Biol. Reprod. 56, 954–963 (1997).
Melman, A., Bar-Chama, N., McCullough, A., Davies, K. & Christ, G. The first human trial for gene transfer therapy for the treatment of erectile dysfunction: preliminary results. Eur. Urol. 48, 314–318 (2005).
Melman, A., Bar-Chama, N., McCullough, A., Davies, K. & Christ, G. hMaxi-K gene transfer in males with erectile dysfunction: results of the first human trial. Hum. Gene Ther. 17, 1165–1176 (2006).
Harraz, A., Shindel, A. W. & Lue, T. F. Emerging gene and stem cell therapies for the treatment of erectile dysfunction. Nat. Rev. Urol. 7, 143–152 (2010).
Bivalacqua, T. J., Burnett, A. L., Hellstrom, W. J. G. & Champion, H. C. Overexpression of arginase in the aged mouse penis impairs erectile function and decreases eNOS activity: influence of in vivo gene therapy of anti-arginase. Am. J. Physiol. Heart Circ. Physiol. 292, H1340–H1351 (2007).
Bivalacqua, T. J., Hellstrom, W. J., Kadowitz, P. J. & Champion, H. C. Increased expression of arginase II in human diabetic corpus cavernosum: in diabetic-associated erectile dysfunction. Biochem. Biophys. Res. Commun. 283, 923–927 (2001).
Bivalacqua, T. J. et al. Adenoviral gene transfer of endothelial nitric oxide synthase (eNOS) to the penis improves age-related erectile dysfunction in the rat. Int. J. Impot. Res. 12 (Suppl. 3), S8–S17 (2000).
Albersen, M. et al. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J. Sex. Med. 7, 3331–3340 (2010).
Yoshimura, N., Kato, R., Chancellor, M. B., Nelson, J. B. & Glorioso, J. C. Gene therapy as future treatment of erectile dysfunction. Expert Opin. Biol. Ther. 10, 1305–1314 (2010).
Magee, T. R. et al. Gene therapy of erectile dysfunction in the rat with penile neuronal nitric oxide synthase. Biol. Reprod. 67, 1033–1041 (2002).
Bivalacqua, T. J. et al. Mesenchymal stem cells alone or ex vivo gene modified with endothelial nitric oxide synthase reverse age-associated erectile dysfunction. Am. J. Physiol. Heart Circ. Physiol. 292, H1278–H1290 (2007).
Chancellor, M. B. et al. Nitric oxide synthase gene transfer for erectile dysfunction in a rat model. BJU Int. 91, 691–696 (2003).
Tirney, S. et al. Nitric oxide synthase gene therapy for erectile dysfunction: comparison of plasmid, adenovirus, and adenovirus-transduced myoblast vectors. Mol. Urol. 5, 37–43 (2001).
Magee, T. R. et al. Protein inhibitor of nitric oxide synthase (NOS) and the N-methyl-D-aspartate receptor are expressed in the rat and mouse penile nerves and colocalize with penile neuronal NOS 1. Biol. Reprod. 488, 478–488 (2003).
Magee, T. R. et al. Antisense and short hairpin RNA (shRNA) constructs targeting PIN (protein inhibitor of NOS) ameliorate aging-related erectile dysfunction in the rat. J. Sex. Med. 4, 633–643 (2007).
Bivalacqua, T. J. et al. Dysregulation of cGMP-dependent protein kinase 1 (PKG-1) impairs erectile function in diabetic rats: influence of in vivo gene therapy of PKG1alpha. BJU Int. 99, 1488–1494 (2007).
Chang, S. et al. Downregulation of cGMP-dependent protein kinase-1 activity in the corpus cavernosum smooth muscle of diabetic rabbits. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R950–R960 (2004).
Das, A., Smolenski, A., Lohmann, S. M. & Kukreja, R. C. Cyclic GMP-dependent protein kinase Ialpha attenuates necrosis and apoptosis following ischemia/reoxygenation in adult cardiomyocyte. J. Biol. Chem. 281, 38644–38652 (2006).
Bakircioglu, M. E. et al. The effect of adeno-associated virus mediated brain derived neurotrophic factor in an animal model of neurogenic impotence. J. Urol. 165, 2103–2109 (2001).
Gholami, S. S. et al. The effect of vascular endothelial growth factor and adeno-associated virus mediated brain derived neurotrophic factor on neurogenic and vasculogenic erectile dysfunction induced by hyperlipidemia. J. Urol. 169, 1577–1581 (2003).
Kato, R. et al. Herpes simplex virus vector-mediated delivery of neurturin rescues erectile dysfunction of cavernous nerve injury. Gene Ther. 16, 26–33 (2009).
Kato, R. et al. Herpes simplex virus vector-mediated delivery of glial cell line-derived neurotrophic factor rescues erectile dysfunction following cavernous nerve injury. Gene Ther. 14, 1344–1352 (2007).
Bennett, N. E. et al. Improvement in erectile dysfunction after neurotrophic factor gene therapy in diabetic rats. J. Urol. 173, 1820–1824 (2005).
Bochinski, D. et al. Effect of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 complex in cavernous nerve cryoablation. Int. J. Impot. Res. 16, 418–423 (2004).
Gerber, H. P., Dixit, V. & Ferrara, N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J. Biol. Chem. 273, 13313–13316 (1998).
Ferrara, N. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. (Berl). 77, 527–543 (1999).
Lin, G. et al. Neurotrophic effects of vascular endothelial growth factor and neurotrophins on cultured major pelvic ganglia. BJU Int. 92, 631–635 (2003).
Dall'Era, J. E. et al. Vascular endothelial growth factor (VEGF) gene therapy using a nonviral gene delivery system improves erectile function in a diabetic rat model. Int. J. Impot. Res. 20, 307–314 (2008).
Rogers, R. S., Graziottin, T. M., Lin, C.-S., Kan, Y. W. & Lue, T. F. Intracavernosal vascular endothelial growth factor (VEGF) injection and adeno-associated virus-mediated VEGF gene therapy prevent and reverse venogenic erectile dysfunction in rats. Int. J. Impot. Res. 15, 26–37 (2003).
Ryu, J.-K. et al. Combined angiopoietin-1 and vascular endothelial growth factor gene transfer restores cavernous angiogenesis and erectile function in a rat model of hypercholesterolemia. Mol. Ther. 13, 705–715 (2006).
Qiu, X. et al. Combined strategy of mesenchymal stem cell injection with vascular endothelial growth factor gene therapy for the treatment of diabetes-associated erectile dysfunction. J. Androl. 33, 37–44 (2012).
Ryu, J.-K. et al. Gene therapy with an erythropoietin enhancer-mediated hypoxia-inducible gene expression system in the corpus cavernosum of mice with high-cholesterol diet-induced erectile dysfunction. J. Androl. doi:10.2164/jandrol.111.016014.
Selvaraju, V. et al. Diabetes, oxidative stress, molecular mechanism, and cardiovascular disease - an overview. Toxicol. Mech. Methods 22, 330–335 (2012).
Bivalacqua, T. J. et al. Superoxide anion production in the rat penis impairs erectile function in diabetes: influence of in vivo extracellular superoxide dismutase gene therapy. J. Sex. Med. 2, 187–198 (2005).
Jin, L. et al. Elevated RhoA/Rho-kinase activity in the aged rat penis: mechanism for age-associated erectile dysfunction. FASEB J. 20, 536–538 (2006).
Gratzke, C. et al. Activated RhoA/Rho kinase impairs erectile function after cavernous nerve injury in rats. J. Urol. 184, 2197–2204 (2010).
Coleman, M. L. et al. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat. Cell Biol. 3, 339–345 (2001).
Bivalacqua, T. J. et al. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc. Natl Acad. Sci. USA 101, 9121–9126 (2004).
Chitaley, K. et al. Adeno-associated viral gene transfer of dominant negative RhoA enhances erectile function in rats. Biochem. Biophys. Res. Commun. 298, 427–432 (2002).
Shen, Z.-J. et al. Gene transfer of vasoactive intestinal polypeptide into the penis improves erectile response in the diabetic rat. BJU Int. 95, 890–894 (2005).
Bivalacqua, T. J., Champion, H. C., Abdel-Mageed, A. B., Kadowitz, P. J. & Hellstrom, W. J. Gene transfer of prepro-calcitonin gene-related peptide restores erectile function in the aged rat. Biol. Reprod. 65, 1371–1377 (2001).
Kendirci, M., Teloken, P. E., Champion, H. C., Hellstrom, W. J. G. & Bivalacqua, T. J. Gene therapy for erectile dysfunction: fact or fiction? Eur. Urol. 50, 1208–1222 (2006).
Christ, G. J. et al. Intracorporal injection of hSlo cDNA restores erectile capacity in STZ-diabetic F-344 rats in vivo. Am. J. Physiol. Heart Circ. Physiol. 287, H1544–H1553 (2004).
Christ, G. J., Spray, D. C. & Brink, P. R. Characterization of K currents in cultured human corporal smooth muscle cells. J. Androl. 14, 319–328.
Christ, G. J. et al. Intracorporal injection of hSlo cDNA in rats produces physiologically relevant alterations in penile function. Am. J. Physiol. 275, H600–H608 (1998).
Melman, A., Zhao, W., Davies, K. P., Bakal, R. & Christ, G. J. The successful long-term treatment of age related erectile dysfunction with hSlo cDNA in rats in vivo. J. Urol. 170, 285–290 (2003).
Melman, A. et al. Gene transfer with a vector expressing Maxi-K from a smooth muscle-specific promoter restores erectile function in the aging rat. Gene Ther. 15, 364–370 (2008).
Christ, G. J. et al. Editorial comment on: Smooth-muscle-specific gene transfer with the human maxi-K channel improves erectile function and enhances sexual behavior in atherosclerotic cynomolgus monkeys. Eur. Urol. 56, 1066 (2009).
Melman, A. & Davies, K. P. Gene therapy in the management of erectile dysfunction (ED): past, present, and future. ScientificWorldJournal 9, 846–854 (2009).
Albersen, M. et al. Multipotent stromal cell therapy for cavernous nerve injury-induced erectile dysfunction. J. Sex. Med. 9, 385–403 (2012).
Bochinski, D. et al. The effect of neural embryonic stem cell therapy in a rat model of cavernosal nerve injury. BJU Int. 94, 904–909 (2004).
Kendirci, M. et al. Transplantation of nonhematopoietic adult bone marrow stem/progenitor cells isolated by p75 nerve growth factor receptor into the penis rescues erectile function in a rat model of cavernous nerve injury. J. Urol. 184, 1560–1566 (2010).
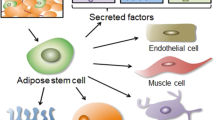
Baraniak, P. R. & McDevitt, T. C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 5, 121–143 (2010).
Zhang, H. et al. Adipose tissue-derived stem cells secrete CXCL5 cytokine with neurotrophic effects on cavernous nerve regeneration. J. Sex. Med. 8, 437–446 (2011).
Albersen, M. & Lue, T. F. Re: Transplantation of nonhematopoietic adult bone marrow stem/progenitor cells isolated by p75 nerve growth factor receptor into the penis rescues erectile function in a rat model of cavernous nerve injury. M. Kendirci, L. Trost, B. Bakondi, M. J. Whitney, W. J. G. Hellstrom and J. L. Spees J Urol 2010; 184: 1560–1566. J. Urol. 185, 1158–1161 (2011).
Lin, G. et al. Tracking intracavernously injected adipose-derived stem cells to bone marrow. Int. J. Impot. Res. 23, 268–275 (2011).
Fandel, T. M. et al. Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur. Urol. 61, 201–210 (2011).
Qiu, X. et al. Effects of Intravenous injection of adipose-derived stem cells in a rat model of radiation therapy-induced erectile dysfunction. J. Sex. Med. http://dx.doi.org/10.1111/j.1743–61092012.02753.x.
Qiu, X. et al. Both immediate and delayed intracavernous injection of autologous adipose-derived stromal vascular fraction enhances recovery of erectile function in a rat model of cavernous nerve injury. Eur. Urol. http://dx.doi.org/10.1016/j.eururo.2012.02.003.
Abdel Aziz, M. T. et al. Effect of mesenchymal stem cell penile transplantation on erectile signaling of aged rats. Andrologia 42, 187–192 (2010).
Qiu, X. et al. Intracavernous transplantation of bone marrow-derived mesenchymal stem cells restores erectile function of streptozocin-induced diabetic rats. J. Sex. Med. 8, 427–436 (2011).
Sun, C. et al. Neurotrophic effect of bone marrow mesenchymal stem cells for erectile dysfunction in diabetic rats. Int. J. Androl. http://dx.doi.org/10.1111/j.1365-26052012.01250.x.
Huang, Y.-C. et al. The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J. Sex. Med. 7, 1391–1400 (2010).
Garcia, M. M. et al. Treatment of erectile dysfunction in the obese type 2 diabetic ZDF rat with adipose tissue-derived stem cells. J. Sex. Med. 7, 89–98 (2010).
Nolazco, G. et al. Effect of muscle-derived stem cells on the restoration of corpora cavernosa smooth muscle and erectile function in the aged rat. BJU Int. 101, 1156–1164 (2008).
Kim, Y. et al. Injection of skeletal muscle-derived cells into the penis improves erectile function. Int. J. Impot. Res. 18, 329–334 (2006).
Woo, J. C. et al. Transplantation of muscle-derived stem cells into the corpus cavernosum restores erectile function in a rat model of cavernous nerve injury. Korean J. Urol. 52, 359–363 (2011).
Carr, L. K. et al. 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int. Urogynecol. J. Pelvic Floor Dysfunct. 19, 881–883 (2008).
Kershen, R. T., Yoo, J. J., Moreland, R. B., Krane, R. J. & Atala, A. Reconstitution of human corpus cavernosum smooth muscle in vitro and in vivo. Tissue Eng. 8, 515–524 (2002).
Park, H. J., Yoo, J. J., Kershen, R. T., Moreland, R. & Atala, A. Reconstitution of human corporal smooth muscle and endothelial cells in vivo. J. Urol. 162, 1106–1109 (1999).
Falke, G., Yoo, J. J., Kwon, T. G., Moreland, R. & Atala, A. Formation of corporal tissue architecture in vivo using human cavernosal muscle and endothelial cells seeded on collagen matrices. Tissue Eng. 9, 871–879 (2003).
Song, L.-J. et al. Construction of cavernosum smooth muscle using umbilical artery smooth muscle cells seeded on acellular corporal collagen matrices. Int. J. Androl. 32, 514–523 (2009).
Chen, K.-L., Eberli, D., Yoo, J. J. & Atala, A. Bioengineered corporal tissue for structural and functional restoration of the penis. Proc. Natl Acad. Sci. USA 107, 3346–3350 (2010).
Ji, C. et al. Construction of tissue-engineered corpus cavernosum with muscle-derived stem cells and transplantation in vivo. BJU Int. 107, 1638–1646 (2011).
Levine, L. A. Editorial comment on: surgical treatment of Peyronie's disease by plaque incision and grafting with buccal mucosa. Eur. Urol. 55, 1475–1476 (2009).
Ferretti, L. et al. Tissue engineering for penile surgery: comparative study of noncellular and cell-seeded synthetic grafts for tunica albuginea replacement. J. Sex. Med. 9, 625–631 (2012).
Imbeault, A. et al. Surgical option for the correction of Peyronie's disease: an autologous tissue-engineered endothelialized graft. J. Sex. Med. 8, 3227–3235 (2011).
Kim, E. D. et al. Interposition of sural nerve restores function of cavernous nerves resected during radical prostatectomy. J. Urol. 161, 188–192 (1999).
Davis, J. W. et al. Randomized phase II trial evaluation of erectile function after attempted unilateral cavernous nerve-sparing retropubic radical prostatectomy with versus without unilateral sural nerve grafting for clinically localized prostate cancer. Eur. Urol. 55, 1135–1143 (2009).
May, F. et al. Nerve replacement strategies for cavernous nerves. Eur. Urol. 48, 372–378 (2005).
Hisasue, S.-I. et al. Cavernous nerve reconstruction with a biodegradable conduit graft and collagen sponge in the rat. J. Urol. 173, 286–291 (2005).
Matsuura, S., Obara, T., Tsuchiya, N., Suzuki, Y. & Habuchi, T. Cavernous nerve regeneration by biodegradable alginate gel sponge sheet placement without sutures. Urology 68, 1366–1371 (2006).
May, F. et al. Schwann cell seeded guidance tubes restore erectile function after ablation of cavernous nerves in rats. J. Urol. 172, 374–377 (2004).
May, F. et al. GDNF-transduced Schwann cell grafts enhance regeneration of erectile nerves. Eur. Urol. 54, 1179–1187 (2008).
Lin, G. et al. Cavernous nerve repair with allogenic adipose matrix and autologous adipose-derived stem cells. Urology 77, 1509.e1–e8 (2011).
Angeloni, N. L. et al. Regeneration of the cavernous nerve by Sonic hedgehog using aligned peptide amphiphile nanofibers. Biomaterials 32, 1091–1101 (2011).
Champion, H. C. et al. Gene transfer of endothelial nitric oxide synthase to the penis augments erectile responses in the aged rat. Proc. Natl Acad. Sci. USA 96, 11648–11652 (1999).
Bivalacqua, T. J. et al. Gene transfer of endothelial nitric oxide synthase partially restores nitric oxide synthesis and erectile function in streptozotocin diabetic rats. J. Urol. 169, 1911–1917 (2003).
Chancellor, M. B. et al. Nitric oxide synthase gene transfer for erectile dysfunction in a rat model. Plasmid 91, 691–696 (2003).
So, I., Chae, M. R. & Lee, S. W. Gene transfer of the K(ATP) channel restores age-related erectile dysfunction in rats. BJU Int. 100, 1154–1160 (2007).
Pu, X-yong, Hu, L-quan, Wang, H.-P., Luo, Y.-X. & Wang, X-huan. Improvement in erectile dysfunction after insulin-like growth factor-1 gene therapy in diabetic rats. Asian J. Androl. 9, 83–91 (2007).
Abdel Aziz, M. T. et al. Effect of HO-1 cDNA-liposome complex transfer on erectile signalling of aged rats. Andrologia 41, 176–183 (2009).
Song, Y. S. et al. Potential differentiation of human mesenchymal stem cell transplanted in rat corpus cavernosum toward endothelial or smooth muscle cells. Int. J. Impot. Res. 19, 378–385 (2007).
Song, Y. S. et al. Human neural crest stem cells transplanted in rat penile corpus cavernosum to repair erectile dysfunction. BJU Int. 102, 220–224 (2008).
Fall, P. A. et al. Apoptosis and effects of intracavernous bone marrow cell injection in a rat model of postprostatectomy erectile dysfunction. Eur. Urol. 56, 716–725 (2009).
Acknowledgements
M. Albersen is a fellow of Research Foundation-Flanders and of the Federico Foundation.
Author information
Authors and Affiliations
Contributions
L. Hakim and M. Albersen researched data for the article and wrote the manuscript. All authors made a substantial contribution to discussion of content and reviewed and edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Hakim, L., Van der Aa, F., Bivalacqua, T. et al. Emerging tools for erectile dysfunction: a role for regenerative medicine. Nat Rev Urol 9, 520–536 (2012). https://doi.org/10.1038/nrurol.2012.143
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2012.143
This article is cited by
-
Influencing Factors for Erectile Dysfunction of Young Adults with No Response to PDE5i
Current Medical Science (2021)
-
Towards clinical application of tissue engineering for erectile penile regeneration
Nature Reviews Urology (2019)
-
What Is the Future of Erectile Dysfunction Therapy?
Current Sexual Health Reports (2018)
-
Erectile dysfunction
Nature Reviews Disease Primers (2016)