Key Points
-
Radiotherapy is integral to the management of many paediatric CNS tumours, but exposes healthy tissue to unwanted radiation that leads to acute and long-term toxicities
-
Continued improvement in long-term survivorship of certain paediatric CNS tumours means that strategies to avoid radiotherapy-related toxicities can improve quality of life and overall functional status of survivors
-
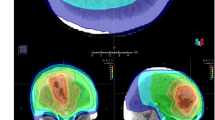
Proton therapy reduces the unwanted, toxicity-associated low-to-intermediate radiation dose that healthy tissue receives in modern X-ray therapy
-
The improvement in dose distribution achieved with proton therapy can meaningfully affect the risk of long-term radiotherapy effects, such as secondary malignancy, cognitive toxicity, endocrinopathy, hearing loss and vasculopathic effects
-
Ongoing research aims to characterize the differential relative biological effectiveness of proton therapy, which has been suggested to increase the risk of imaging changes in susceptible structures, such as the brainstem
-
Despite its higher up-front costs, proton therapy has been shown to be more cost effective than X-ray therapy owing to the dramatic reduction in the excess costs of managing long-term toxicities
Abstract
Radiotherapy is an integral and highly effective aspect of the management of many paediatric CNS tumours, including embryonal tumours, astrocytic tumours and ependymal tumours. Nevertheless, continued improvements in long-term survivorship of such tumours means that radiotherapy-related toxicities that affect quality of life and overall functional status for survivors are increasingly problematic, and strategies that mitigate these adverse effects are needed. One such strategy is proton therapy, which has distinct advantages over conventional photon therapy and enables greater precision in the delivery of tumoricidal radiation doses with reduced irradiation of healthy tissues. These dose distribution advantages can translate into clinical benefits by reducing the risk of long-term adverse effects of radiotherapy, such as secondary malignancy, cognitive toxicity, endocrinopathy, hearing loss and vasculopathic effects. As the availability of proton therapy increases with the development of new proton centres, this treatment modality is increasingly being used in the management of paediatric CNS tumours. In this Review, we provide an introduction to the types of paediatric CNS tumours for which proton therapy can be considered, and discuss the available evidence that proton therapy limits toxicities and improves quality of life for patients. We will also consider uncertainties surrounding the use of proton therapy, evidence for its cost-effectiveness, and its future role in the management of paediatric CNS tumours.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Oeffinger, K. C. et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 355, 1572–1582 (2006).
Indelicato, D. & Chang, A. Pediatric proton therapy: patterns of care in 2013 across the United States [abstract 029]. Int. J. Part. Ther. 1, 772 (2014).
Freeman, C. R., Farmer, J.-P. & Taylor, R. E. Perez and Brady's Principles and Practice of Radiation Oncology (eds Halperin, E. C. et al.) (Lippincott Williams & Wilkins, 2013).
Ostrom, Q. T. et al. Alex's Lemonade Stand Foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 16 (Suppl. 10), ×1–×36 (2015).
Kohler, B. A. et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J. Natl Cancer Inst. 103, 714–736 (2011).
Merchant, T. E. et al. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J. Clin. Oncol. 27, 3598–3604 (2009).
Merchant, T. E. et al. Phase II trial of conformal radiation therapy for pediatric patients with craniopharyngioma and correlation of surgical factors and radiation dosimetry with change in cognitive function. J. Neurosurg. 104, 94–102 (2006).
Merchant, T. E. et al. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 10, 258–266 (2009).
Aoyama, H. et al. Induction chemotherapy followed by low-dose involved-field radiotherapy for intracranial germ cell tumors. J. Clin. Oncol. 20, 857–865 (2002).
Mariotto, A. B. et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol. Biomarkers Prev. 18, 1033–1040 (2009).
Bouffet, E. et al. M4 protocol for cerebellar medulloblastoma: supratentorial radiotherapy may not be avoided. Int. J. Radiat. Oncol. Biol. Phys. 24, 79–85 (1992).
Thomas, P. R. et al. Low-stage medulloblastoma: final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. J. Clin. Oncol. 18, 3004–3011 (2000).
Packer, R. J. et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J. Clin. Oncol. 24, 4202–4208 (2006).
Packer, R. J. et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: a Children's Cancer Group study. J. Clin. Oncol. 17, 2127–2136 (1999).
Duffner, P. K. et al. The treatment of malignant brain tumors in infants and very young children: an update of the Pediatric Oncology Group experience. Neuro Oncol. 1, 152–161 (1999).
Woehrer, A. et al. Incidence of atypical teratoid/rhabdoid tumors in children: a population-based study by the Austrian Brain Tumor Registry, 1996–2006. Cancer 116, 5725–5732 (2010).
Tekautz, T. M. et al. Atypical teratoid/rhabdoid tumors (ATRT): improved survival in children 3 years of age and older with radiation therapy and high-dose alkylator-based chemotherapy. J. Clin. Oncol. 23, 1491–1499 (2005).
Wisoff, J. H. et al. Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children's Oncology Group. Neurosurgery 68, 1548–1554 (2011).
Saunders, D. E., Phipps, K. P., Wade, A. M. & Hayward, R. D. Surveillance imaging strategies following surgery and/or radiotherapy for childhood cerebellar low-grade astrocytoma. J. Neurosurg. 102, 172–178 (2005).
Goldwein, J. W. et al. Is craniospinal irradiation required to cure children with malignant (anaplastic) intracranial ependymomas? Cancer 67, 2766–2771 (1991).
Merchant, T. E., Haida, T., Wang, M. H., Finlay, J. L. & Leibel, S. A. Anaplastic ependymoma: treatment of pediatric patients with or without craniospinal radiation therapy. J. Neurosurg. 86, 943–949 (1997).
Timmermann, B. et al. Combined postoperative irradiation and chemotherapy for anaplastic ependymomas in childhood: results of the German prospective trials HIT 88/89 and HIT 91. Int. J. Radiat. Oncol. Biol. Phys. 46, 287–295 (2000).
Timmermann, B. et al. Role of radiotherapy in anaplastic ependymoma in children under age of 3 years: results of the prospective German brain tumor trials HIT-SKK 87 and 92. Radiother. Oncol. 77, 278–285 (2005).
van Veelen-Vincent, M. L. et al. Ependymoma in childhood: prognostic factors, extent of surgery, and adjuvant therapy. J. Neurosurg. 97, 827–835 (2002).
Duffner, P. K. et al. Prognostic factors in infants and very young children with intracranial ependymomas. Pediatr. Neurosurg. 28, 215–222 (1998).
Grill, J. et al. Postoperative chemotherapy without irradiation for ependymoma in children under 5 years of age: a multicenter trial of the French Society of Pediatric Oncology. J. Clin. Oncol. 19, 1288–1296 (2001).
Massimino, M. et al. Salvage treatment for childhood ependymoma after surgery only: pitfalls of omitting 'at once' adjuvant treatment. Int. J. Radiat. Oncol. Biol. Phys. 65, 1440–1445 (2006).
Massimino, M. et al. Infant ependymoma in a 10-year AIEOP (Associazione Italiana Ematologia Oncologia Pediatrica) experience with omitted or deferred radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 80, 807–814 (2011).
Merchant, T. E. et al. A phase II trial of conformal radiation therapy for pediatric patients with localized ependymoma, chemotherapy prior to second surgery for incompletely resected ependymoma, and observation for completely resected, differentiated, supratentorial ependymoma. Int. J. Radiat. Oncol. Biol. Phys. 93, S1 (2015).
[No authors listed.] Prescribing, reporting and recording proton beam therapy. J ICRU 7, NP (2007).
Eaton, B. R. et al. Clinical outcomes among children with standard-risk medulloblastoma treated with proton and photon radiation therapy: a comparison of disease control and overall survival. Int. J. Radiat. Oncol. Biol. Phys. 94, 133–138 (2016).
Mostow, E. N., Byrne, J., Connelly, R. R. & Mulvihill, J. J. Quality of life in long-term survivors of CNS tumors of childhood and adolescence. J. Clin. Oncol. 9, 592–599 (1991).
Robinson, K. E. et al. A quantitative meta-analysis of neurocognitive sequelae in survivors of pediatric brain tumors. Pediatr. Blood Cancer 55, 525–531 (2010).
Mulhern, R. K., Hancock, J., Fairclough, D. & Kun, L. Neuropsychological status of children treated for brain tumors: a critical review and integrative analysis. Med. Pediatr. Oncol. 20, 181–191 (1992).
Reddick, W. E. et al. A hybrid neural network analysis of subtle brain volume differences in children surviving brain tumors. Magn. Reson. Imaging 16, 413–421 (1998).
Walter, A. W. et al. Survival and neurodevelopmental outcome of young children with medulloblastoma at St. Jude Children's Research Hospital. J. Clin. Oncol. 17, 3720–3728 (1999).
Ris, M. D., Packer, R., Goldwein, J., Jones-Wallace, D. & Boyett, J. M. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children's Cancer Group study. J. Clin. Oncol. 19, 3470–3476 (2001).
Moxon-Emre, I. et al. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J. Clin. Oncol. 32, 1760–1768 (2014).
Moxon-Emre, I. et al. Vulnerability of white matter to insult during childhood: evidence from patients treated for medulloblastoma. J. Neurosurg. Pediatr. 25, 1–12 (2016).
Duffner, P. K. Risk factors for cognitive decline in children treated for brain tumors. Eur. J. Paediatr. Neurol. 14, 106–115 (2010).
Padovani, L., Andre, N., Constine, L. S. & Muracciole, X. Neurocognitive function after radiotherapy for paediatric brain tumours. Nat. Rev. Neurol. 8, 578–588 (2012).
Palmer, S. L. et al. Processing speed, attention, and working memory after treatment for medulloblastoma: an international, prospective, and longitudinal study. J. Clin. Oncol. 31, 3494–3500 (2013).
Conklin, H. M. et al. Long-term efficacy of methylphenidate in enhancing attention regulation, social skills, and academic abilities of childhood cancer survivors. J. Clin. Oncol. 28, 4465–4472 (2010).
Mulhern, R. K. et al. Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. J. Clin. Oncol. 19, 472–479 (2001).
Fry, A. F. & Hale, S. Relationships among processing speed, working memory, and fluid intelligence in children. Biol. Psychol. 54, 1–34 (2000).
Mulhern, R. K. et al. Neurocognitive deficits in medulloblastoma survivors and white matter loss. Ann. Neurol. 46, 834–841 (1999).
Liu, A. K. et al. Changes in cerebral cortex of children treated for medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 68, 992–998 (2007).
Gondi, V., Tome, W. A. & Mehta, M. P. Why avoid the hippocampus? A comprehensive review. Radiother. Oncol. 97, 370–376 (2010).
Redmond, K. J. et al. Association between radiation dose to neuronal progenitor cell niches and temporal lobes and performance on neuropsychological testing in children: a prospective study. Neuro Oncol. 15, 360–369 (2013).
Merchant, T. E. et al. Critical combinations of radiation dose and volume predict intelligence quotient and academic achievement scores after craniospinal irradiation in children with medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 90, 554–561 (2014).
Merchant, T. E. et al. Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr. Blood Cancer 51, 110–117 (2008).
Pulsifer, M. B. et al. Early cognitive outcomes following proton radiation in pediatric patients with brain and central nervous system tumors. Int. J. Radiat. Oncol. Biol. Phys. 93, 400–407 (2015).
Greenberger, B. A. et al. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. Int. J. Radiat. Oncol. Biol. Phys. 89, 1060–1068 (2014).
Yock, T. I. et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol. 17, 287–298 (2016).
Kahalley, L. S. et al. Comparing intelligence quotient change after treatment with proton versus photon radiation therapy for pediatric brain tumors. J. Clin. Oncol. 34, 1043–1049 (2016).
Engelman, A. et al. Implications of pediatric brain radiotherapy: photons, protons, and the probability of requiring special education. [abstract] Int. J. Part. Ther. 2, 333 (2015).
Gurney, J. G. et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer 97, 663–673 (2003).
Darzy, K. H. & Shalet, S. M. Hypopituitarism following radiotherapy. Pituitary 12, 40–50 (2009).
Laughton, S. J. et al. Endocrine outcomes for children with embryonal brain tumors after risk-adapted craniospinal and conformal primary-site irradiation and high-dose chemotherapy with stem-cell rescue on the SJMB-96 trial. J. Clin. Oncol. 26, 1112–1118 (2008).
Ogilvy-Stuart, A. L. et al. Endocrine deficit after fractionated total body irradiation. Arch. Dis. Child. 67, 1107–1110 (1992).
Merchant, T. E. et al. Growth hormone secretion after conformal radiation therapy in pediatric patients with localized brain tumors. J. Clin. Oncol. 29, 4776–4780 (2011).
Vatner, R. et al. Comparison of endocrine dysfunction and dosimetry in pediatric patients treated with proton versus photon radiation therapy for medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 93, S32–S33 (2015).
Macdonald, S. M. et al. Proton radiotherapy for pediatric central nervous system ependymoma: clinical outcomes for 70 patients. Neuro Oncol. 15, 1552–1559 (2013).
De Amorim Bernstein, K. et al. Early clinical outcomes using proton radiation for children with central nervous system atypical teratoid rhabdoid tumors. Int. J. Radiat. Oncol. Biol. Phys. 86, 114–120 (2013).
Brinkman, T. M. et al. Treatment-induced hearing loss and adult social outcomes in survivors of childhood CNS and non-CNS solid tumors: results from the St. Jude Lifetime Cohort Study. Cancer 121, 4053–4061 (2015).
Bass, J. K. et al. Hearing loss in patients who received cranial radiation therapy for childhood cancer. J. Clin. Oncol. 34, 1248–1255 (2016).
Hua, C., Bass, J. K., Khan, R., Kun, L. E. & Merchant, T. E. Hearing loss after radiotherapy for pediatric brain tumors: effect of cochlear dose. Int. J. Radiat. Oncol. Biol. Phys. 72, 892–899 (2008).
Merchant, T. E. et al. Early neuro-otologic effects of three-dimensional irradiation in children with primary brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 58, 1194–1207 (2004).
Li, Y., Womer, R. B. & Silber, J. H. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur. J. Cancer 40, 2445–2451 (2004).
Kretschmar, C. S., Warren, M. P., Lavally, B. L., Dyer, S. & Tarbell, N. J. Ototoxicity of preradiation cisplatin for children with central nervous system tumors. J. Clin. Oncol. 8, 1191–1198 (1990).
Moeller, B. J. et al. Low early ototoxicity rates for pediatric medulloblastoma patients treated with proton radiotherapy. Radiat. Oncol. 6, 58 (2011).
Jimenez, R. B. et al. Proton radiation therapy for pediatric medulloblastoma and supratentorial primitive neuroectodermal tumors: outcomes for very young children treated with upfront chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 87, 120–126 (2013).
Passos, J. et al. Late cerebrovascular complications after radiotherapy for childhood primary central nervous system tumors. Pediatr. Neurol. 53, 211–215 (2015).
Haddy, N. et al. Relationship between the brain radiation dose for the treatment of childhood cancer and the risk of long-term cerebrovascular mortality. Brain 134, 1362–1372 (2011).
Omura, M., Aida, N., Sekido, K., Kakehi, M. & Matsubara, S. Large intracranial vessel occlusive vasculopathy after radiation therapy in children: clinical features and usefulness of magnetic resonance imaging. Int. J. Radiat. Oncol. Biol. Phys. 38, 241–249 (1997).
Bowers, D. C. et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 24, 5277–5282 (2006).
Darby, S. C. et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 368, 987–998 (2013).
van Nimwegen, F. A. et al. Radiation dose–response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J. Clin. Oncol. 34, 235–243 (2015).
Jakacki, R. I., Goldwein, J. W., Larsen, R. L., Barber, G. & Silber, J. H. Cardiac dysfunction following spinal irradiation during childhood. J. Clin. Oncol. 11, 1033–1038 (1993).
Boehling, N. S. et al. Dosimetric comparison of three-dimensional conformal proton radiotherapy, intensity-modulated proton therapy, and intensity-modulated radiotherapy for treatment of pediatric craniopharyngiomas. Int. J. Radiat. Oncol. Biol. Phys. 82, 643–652 (2012).
Zhang, R. et al. A comparative study on the risks of radiogenic second cancers and cardiac mortality in a set of pediatric medulloblastoma patients treated with photon or proton craniospinal irradiation. Radiother. Oncol. 113, 84–88 (2014).
Armstrong, G. T. Long-term survivors of childhood central nervous system malignancies: the experience of the Childhood Cancer Survivor Study. Eur. J. Paediatr. Neurol. 14, 298–303 (2010).
Mertens, A. C. et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J. Natl Cancer Inst. 100, 1368–1379 (2008).
Morris, E. B. et al. Survival and late mortality in long-term survivors of pediatric CNS tumors. J. Clin. Oncol. 25, 1532–1538 (2007).
Peterson, K. M., Shao, C., McCarter, R., MacDonald, T. J. & Byrne, J. An analysis of SEER data of increasing risk of secondary malignant neoplasms among long-term survivors of childhood brain tumors. Pediatr. Blood Cancer 47, 83–88 (2006).
Chung, C. S. et al. Incidence of second malignancies among patients treated with proton versus photon radiation. Int. J. Radiat. Oncol. Biol. Phys. 87, 46–52 (2013).
Moteabbed, M., Yock, T. I. & Paganetti, H. The risk of radiation-induced second cancers in the high to medium dose region: a comparison between passive and scanned proton therapy, IMRT and VMAT for pediatric patients with brain tumors. Phys. Med. Biol. 59, 2883–2899 (2014).
Yock, T. I. & Caruso, P. A. Risk of second cancers after photon and proton radiotherapy: a review of the data. Health Phys. 103, 577–585 (2012).
Yock, T. I. et al. Quality of life outcomes in proton and photon treated pediatric brain tumor survivors. Radiother. Oncol. 113, 89–94 (2014).
Mailhot Vega, R. B. et al. Cost effectiveness of proton therapy compared with photon therapy in the management of pediatric medulloblastoma. Cancer 119, 4299–4307 (2013).
Lundkvist, J., Ekman, M., Ericsson, S. R., Jonsson, B. & Glimelius, B. Proton therapy of cancer: potential clinical advantages and cost-effectiveness. Acta Oncol. 44, 850–861 (2005).
Mailhot Vega, R. et al. Cost effectiveness of proton versus photon radiation therapy with respect to the risk of growth hormone deficiency in children. Cancer 121, 1694–1702 (2015).
Paganetti, H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys. Med. Biol. 59, R419–R472 (2014).
Cuaron, J. J. et al. Exponential increase in relative biological effectiveness along distal edge of a proton Bragg peak as measured by deoxyribonucleic acid double-strand breaks. Int. J. Radiat. Oncol. Biol. Phys. 95, 62–69 (2016).
Gunther, J. R. et al. Imaging changes in pediatric intracranial ependymoma patients treated with proton beam radiation therapy compared to intensity modulated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 93, 54–63 (2015).
Kralik, S. F. et al. Radiation necrosis in pediatric patients with brain tumors treated with proton radiotherapy. AJNR Am. J. Neuroradiol. 36, 1572–1578 (2015).
Sabin, N. D. et al. Imaging changes in very young children with brain tumors treated with proton therapy and chemotherapy. AJNR Am. J. Neuroradiol. 34, 446–450 (2013).
Indelicato, D. J. et al. Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol. 53, 1298–1304 (2014).
Buchsbaum, J. C. et al. Range modulation in proton therapy planning: a simple method for mitigating effects of increased relative biological effectiveness at the end-of-range of clinical proton beams. Radiat. Oncol. 9, 2 (2014).
Giantsoudi, D. et al. Incidence of CNS injury for a cohort of 111 patients treated with proton therapy for medulloblastoma: LET and RBE associations for areas of injury. Int. J. Radiat. Oncol. Biol. Phys. 95, 287–296 (2016).
Underwood, T. et al. Can we advance proton therapy for prostate? Considering alternative beam angles and relative biological effectiveness variations when comparing against intensity modulated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 95, 454–464 (2016).
Polf, J. C., Chuong, M., Zhang, B. & Mehta, M. P. Anteriorly oriented beam arranges with daily in vivo range verification for proton therapy for prostate cancer: rectal toxicity rates. Int. J. Part. Ther. 2, 509–517 (2016).
Rosenblatt, E., Meghzifene, A., Belyakov, O. & Abdel-Wahab, M. Relevance of particle therapy to developing countries. Int. J. Radiat. Oncol. Biol. Phys. 95, 25–29 (2016).
Schlocker, A. J. & Corn, B. W. Proton beam therapy in small nations: financial irrationality or unique collaborative opportunity? Int. J. Radiat. Oncol. Biol. Phys. 95, 21–24 (2016).
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for the article, and provided substantial contribution to discussion of content, writing, reviewing and editing the article.
Corresponding author
Ethics declarations
Competing interests
V.G. has received speaking honoraria from prIME Oncology and US Oncology. None of these activities are related to this paper. M.P.M. has served as a consultant for BMS, Cavion, Celldex, Elekta, Novartis, Novocure and Roche; has previously held stock options in Pharmacyclics where he served on the Board of Directors; and has received research funding from Cellectar, NIH and Novocure. None of these activities are related to this paper. T.I.Y. declares no competing interests.
Rights and permissions
About this article
Cite this article
Gondi, V., Yock, T. & Mehta, M. Proton therapy for paediatric CNS tumours — improving treatment-related outcomes. Nat Rev Neurol 12, 334–345 (2016). https://doi.org/10.1038/nrneurol.2016.70
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2016.70
This article is cited by
-
Oxygen supplementation in anesthesia can block FLASH effect and anti-tumor immunity in conventional proton therapy
Communications Medicine (2023)
-
Mitigating Radiotoxicity in the Central Nervous System: Role of Proton Therapy
Current Treatment Options in Oncology (2023)
-
The incidence of radiation-induced moyamoya among pediatric brain tumor patients who received photon radiation versus those who received proton beam therapy: a systematic review
Neurosurgical Review (2023)
-
Physics and biomedical challenges of cancer therapy with accelerated heavy ions
Nature Reviews Physics (2021)
-
White matter hyperintensity volumes are related to processing speed in long-term survivors of childhood cerebellar tumors
Journal of Neuro-Oncology (2021)