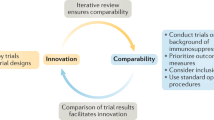
Abstract
The introduction of immunomodulatory treatments has transformed the management of patients with relapsing–remitting multiple sclerosis (MS), but has had no consistent benefit in progressive MS. Patients with primary or secondary progressive MS, therefore, are faced with relentless functional decline that remains without treatment. Clinical trials in progressive MS are clearly needed, but their design and conduct is challenging, and different from that of trials in relapsing–remitting MS. Challenges to reliable measurement of clinical progression, uncertainties about the natural history of progressive MS, and the unclear role of imaging outcomes all impede optimal trial design. Clinical trials in progressive MS have used time to a predefined change on the Expanded Disability Status Scale as their main outcome measure, which has had important consequences for trial duration and has led to inclusion of only a highly selected minority of patients. Here, we review the current approach to clinical trial design in progressive MS, outline key ongoing challenges, and suggest strategies to overcome such hurdles.
Key Points
-
During the course of multiple sclerosis, most patients are affected by a progressive disease course (PMS), for which effective treatments are lacking
-
Treatment targets in PMS are speculative, but could include remyelination, cytoprotection and inhibition of microglial activation
-
Reliable measurement of clinical progression in PMS is challenging, and alternatives to the current outcome measure of change on the Expanded Disability Status Scale are needed
-
Brain atrophy measured on MRI is an attractive potential trial outcome, but its relationship to disease progression remains to be established
-
Stringent inclusion criteria used in current trials in PMS lead to exclusion of the majority of patients with PMS
-
New trial models, such as adaptive and seamless phase II–III designs and the Simon-2-stage model, could help to accelerate development of treatments for PMS
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Compston, A. & Coles, A. Multiple sclerosis. Lancet 359, 1221–1231 (2002).
Miller, D. H. & Leary, S. M. Primary-progressive multiple sclerosis. Lancet Neurol. 6, 903–912 (2007).
Rovaris, M. et al. Secondary progressive multiple sclerosis: current knowledge and future challenges. Lancet Neurol. 5, 343–354 (2006).
[No authors listed]. Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 352, 1498–1504 (1998).
[No authors listed]. Placebo-controlled multicentre randomised trial of interferon β-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon β-1b in secondary progressive MS. Lancet 352, 1491–1497 (1998).
Johnson, K. P. et al. Copolymer 1 reduces relapse rate and improves disability in relapsing–remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 45, 1268–1276 (1995).
Polman, C. H. et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 354, 899–910 (2006).
Killestein, J., Rudick, R. A. & Polman, C. H. Oral treatment for multiple sclerosis. Lancet Neurol. 10, 1026–1034 (2011).
Meuth, S. G., Göbel, K. & Wiendl, H. Immune therapy of multiple sclerosis—future strategies. Curr. Pharm. Des. 18, 4489–4497 (2012).
La Mantia, L., Munari, L. M. & Lovati, R. Glatiramer acetate for multiple sclerosis. Cochrane Database of Systematic Reviews, Issue 12. Art No.: CD004678. http://dx.doi.org/10.1002/14651858.CD004678.pub2.
La Mantia, L. et al. Interferon beta for secondary progressive multiple sclerosis. Cochrane Database of Systematic Reviews, Issue 1. Art. No.: CD005181. http://dx.doi.org/10.1002/14651858.CD005181.pub3.
Bjartmar, C., Wujek, J. R. & Trapp, B. D. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J. Neurol. Sci. 206, 165–171 (2003).
Bjartmar, C. & Trapp, B. D. Axonal degeneration and progressive neurologic disability in multiple sclerosis. Neurotox. Res. 5, 157–164 (2003).
Antel, J., Antel, S., Caramanos, Z., Arnold, D. L. & Kuhlmann, T. Primary progressive multiple sclerosis: part of the MS disease spectrum or separate disease entity? Acta Neuropathol. 123, 627–638 (2012).
Kutzelnigg, A. et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128, 2705–2712 (2005).
Lassmann, H., Van Horssen, J. & Mahad, D. Progressive multiple sclerosis: pathology and pathogenesis. Nat. Rev. Neurol. 8, 647–656 (2012).
Calabrese, M. et al. Cortical lesion load associates with progression of disability in multiple sclerosis. Brain 135, 2952–2961 (2012).
Kurtzke, J. F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33, 1444–1452 (1983).
Leray, E. et al. Evidence for a two-stage disability progression in multiple sclerosis. Brain 133, 1900–1913 (2010).
Scalfari, A. et al. The natural history of multiple sclerosis, a geographically based study 10: relapses and long-term disability. Brain 133, 1914–1929 (2010).
Confavreux, C., Vukusic, S. & Adeleine, P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain 126, 770–782 (2003).
Prineas, J. W. et al. Immunopathology of secondary-progressive multiple sclerosis. Ann. Neurol. 50, 646–657 (2001).
Jackson, S. J., Giovannoni, G. & Baker, D. Fingolimod modulates microglial activation to augment markers of remyelination. J. Neuroinflammation 8, 76 (2011).
Brück, W. et al. Reduced astrocytic NF-κB activation by laquinimod protects from cuprizone-induced demyelination. Acta Neuropathol. 124, 411–424 (2012).
Wilms, H. et al. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1β, TNF-α and IL-6 in an in-vitro model of brain inflammation. J. Neuroinflammation 7, 30 (2010).
Yong, V. W. et al. The promise of minocycline in neurology. Lancet Neurol. 3, 744–751 (2004).
Nikolakopoulou, A. M., Dutta, R., Chen, Z., Miller, R. H. & Trapp, B. D. Activated microglia enhance neurogenesis via trypsinogen secretion. Proc. Natl Acad. Sci. USA http://dx.doi.org/10.1073/pnas.1218856110.
Gonsette, R. E. Neurodegeneration in multiple sclerosis: the role of oxidative stress and excitotoxicity. J. Neurol. Sci. 274, 48–53 (2008).
Salter, M. G. & Fern, R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature 438, 1167–1171 (2005).
Micu, I. et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature 439, 988–992 (2006).
Ouardouz, M. et al. Glutamate receptors on myelinated spinal cord axons: II. AMPA and GluR5 receptors. Ann. Neurol. 65, 160–166 (2009).
Irvine, K. A. & Blakemore, W. F. Remyelination protects axons from demyelination-associated axon degeneration. Brain 131, 1464–1477 (2008).
Rudick, R. A., Mi, S. & Sandrock, A. W. Jr. LINGO-1 antagonists as therapy for multiple sclerosis: in vitro and in vivo evidence. Expert. Opin. Biol. Ther. 8, 1561–1570 (2008).
Keough, M. B. & Yong, V. W. Remyelination therapy for multiple sclerosis. Neurotherapeutics 10, 44–54 (2013).
Madeddu, R. et al. Cytoskeletal proteins in the cerebrospinal fluid as biomarker of multiple sclerosis. Neurol. Sci. 34, 181–186 (2013).
Koch, M. W., Metz, L. M. & Kovalchuk, O. Epigenetic changes in patients with multiple sclerosis. Nat. Rev. Neurol. 9, 35–43 (2013).
Koch, M. W., Metz, L. M. & Kovalchuk, O. Epigenetics and miRNAs in the diagnosis and treatment of multiple sclerosis. Trends Mol. Med. 19, 23–30 (2013).
Noorbakhsh, F. et al. Impaired neurosteroid synthesis in multiple sclerosis. Brain 134, 2703–2721 (2011).
Rajasekharan, S. & Bar-Or, A. From bench to MS bedside: challenges translating biomarker discovery to clinical practice. J. Neuroimmunol. 248, 66–72 (2012).
Wolinsky, J. S. et al. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann. Neurol. 61, 14–24 (2007).
Hommes, O. R. et al. Intravenous immunoglobulin in secondary progressive multiple sclerosis: randomised placebo-controlled trial. Lancet 364, 1149–1156 (2004).
Panitch, H., Miller, A., Paty, D. & Weinshenker, B. Interferon β-1b in secondary progressive MS: results from a 3-year controlled study. Neurology 63, 1788–1795 (2004).
Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon-Beta-1a in MS (SPECTRIMS) Study Group. Randomized controlled trial of interferon- beta-1a in secondary progressive MS: Clinical results. Neurology 56, 1496–1504 (2001).
Bosma, L. V. et al. The search for responsive clinical endpoints in primary progressive multiple sclerosis. Mult. Scler. 15, 715–720 (2009).
Ontaneda, D., Larocca, N., Coetzee, T. & Rudick, R. Revisiting the Multiple Sclerosis Functional Composite: proceedings from the National Multiple Sclerosis Society (NMSS) Task Force on Clinical Disability Measures. Mult. Scler. 18, 1074–1080 (2012).
Amato, M. P., Fratiglioni, L., Groppi, C., Siracusa, G. & Amaducci, L. Interrater reliability in assessing functional systems and disability on the Kurtzke scale in multiple sclerosis. Arch. Neurol. 45, 746–748 (1988).
Francis, D. A., Bain, P., Swan, A. V. & Hughes, R. A. An assessment of disability rating scales used in multiple sclerosis. Arch. Neurol. 48, 299–301 (1991).
Noseworthy, J. H., Vandervoort, M. K., Wong, C. J. & Ebers, G. C. Interrater variability with the Expanded Disability Status Scale (EDSS) and Functional Systems (FS) in a multiple sclerosis clinical trial. The Canadian Cooperation MS Study Group. Neurology 40, 971–975 (1990).
Weinshenker, B. G. et al. The natural history of multiple sclerosis: a geographically based study. 4. Applications to planning and interpretation of clinical therapeutic trials. Brain 114, 1057–1067 (1991).
Cohen, J. A. et al. Benefit of interferon β-1a on MSFC progression in secondary progressive MS. Neurology 59, 679–687 (2002).
Cutter, G. R. et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain 122, 871–882 (1999).
Kapoor, R. et al. Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol. 9, 681–688 (2010).
Hoogervorst, E. L. et al. Comparisons of patient self-report, neurologic examination, and functional impairment in MS. Neurology 56, 934–937 (2001).
Hoogervorst, E. L., Kalkers, N. F., Cutter, G. R., Uitdehaag, B. M. & Polman, C. H. The patient's perception of a (reliable) change in the Multiple Sclerosis Functional Composite. Mult. Scler. 10, 55–60 (2004).
Miller, D. M., Rudick, R. A., Cutter, G., Baier, M. & Fischer, J. S. Clinical significance of the Multiple Sclerosis Functional Composite: relationship to patient-reported quality of life. Arch. Neurol. 57, 1319–1324 (2000).
Solari, A., Radice, D., Manneschi, L., Motti, L. & Montanari, E. The Multiple Sclerosis Functional Composite: different practice effects in the three test components. J. Neurol. Sci. 228, 71–74 (2005).
Schwid, S. R., Goodman, A. D., McDermott, M. P., Bever, C. F. & Cook, S. D. Quantitative functional measures in MS: what is a reliable change? Neurology 58, 1294–1296 (2002).
Bosma, L. V. et al. Progression on the Multiple Sclerosis Functional Composite in multiple sclerosis: what is the optimal cut-off for the three components? Mult. Scler. 16, 862–867 (2010).
Kragt, J. J., van der Linden, F. A., Nielsen, J. M., Uitdehaag, B. M. & Polman, C. H. Clinical impact of 20% worsening on Timed 25-foot Walk and 9-hole Peg Test in multiple sclerosis. Mult. Scler. 12, 594–598 (2006).
Polman, C. H. & Rudick, R. A. The multiple sclerosis functional composite: a clinically meaningful measure of disability. Neurology 74 (Suppl. 3), S8–S15 (2010).
Tombaugh, T. N. A comprehensive review of the Paced Auditory Serial Addition Test (PASAT). Arch. Clin. Neuropsychol. 21, 53–76 (2006).
Scherer, P. Cognitive screening in multiple sclerosis. J. Neurol. 254 (Suppl. 2), II26–II29 (2007).
Parmenter, B. A., Weinstock-Guttman, B., Garg, N., Munschauer, F. & Benedict, R. H. Screening for cognitive impairment in multiple sclerosis using the Symbol Digit Modalities Test. Mult. Scler. 13, 52–57 (2007).
Morrow, S. A. et al. Predicting loss of employment over three years in multiple sclerosis: clinically meaningful cognitive decline. Clin. Neuropsychol. 24, 1131–1145 (2010).
Balcer, L. J. & Frohman, E. M. Evaluating loss of visual function in multiple sclerosis as measured by low-contrast letter acuity. Neurology 74 (Suppl. 3), S16–S23 (2010).
Balcer, L. J. et al. New low-contrast vision charts: reliability and test characteristics in patients with multiple sclerosis. Mult. Scler. 6, 163–171 (2000).
Mowry, E. M. et al. Vision related quality of life in multiple sclerosis: correlation with new measures of low and high contrast letter acuity. J. Neurol. Neurosurg. Psychiatry 80, 767–772 (2009).
Frohman, E. M. et al. Characterizing the mechanisms of progression in multiple sclerosis: evidence and new hypotheses for future directions. Arch. Neurol. 62, 1345–1356 (2005).
Daumer, M., Neuhaus, A., Morrissey, S., Hintzen, R. & Ebers, G. C. MRI as an outcome in multiple sclerosis clinical trials. Neurology 72, 705–711 (2009).
Geurts, J. J., Calabrese, M., Fisher, E. & Rudick, R. A. Measurement and clinical effect of grey matter pathology in multiple sclerosis. Lancet Neurol. 11, 1082–1092 (2012).
Altmann, D. R. et al. Sample sizes for brain atrophy outcomes in trials for secondary progressive multiple sclerosis. Neurology 72, 595–601 (2009).
Cohen, J. A., Reingold, S. C., Polman, C. H. & Wolinsky, J. S. Disability outcome measures in multiple sclerosis clinical trials: current status and future prospects. Lancet Neurol. 11, 467–476 (2012).
Havrdova, E. et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol. 8, 254–260 (2009).
Giovannoni, G. et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol. 10, 329–337 (2011).
Cottrell, D. A. et al. The natural history of multiple sclerosis: a geographically based study. 5. The clinical features and natural history of primary progressive multiple sclerosis. Brain 122, 625–639 (1999).
Confavreux, C., Vukusic, S., Moreau, T. & Adeleine, P. Relapses and progression of disability in multiple sclerosis. N. Engl. J. Med. 343, 1430–1438 (2000).
Koch, M., Kingwell, E., Rieckmann, P. & Tremlett, H. The natural history of primary progressive multiple sclerosis. Neurology 73, 1996–2002 (2009).
Tremlett, H., Paty, D. & Devonshire, V. Disability progression in multiple sclerosis is slower than previously reported. Neurology 66, 172–177 (2006).
US National Institutes of Health. FTY720 in patients with primary progressive multiple sclerosis. ClinicalTrials.gov [online].
Mostert, J. P., Koch, M. W., Heerings, M., Heersema, D. J. & De Keyser, J. Therapeutic potential of fluoxetine in neurological disorders. CNS Neurosci. Ther. 14, 153–164 (2008).
Sicotte, N. L. et al. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann. Neurol. 52, 421–428 (2002).
Garay, L. et al. Protective effects of progesterone administration on axonal pathology in mice with experimental autoimmune encephalomyelitis. Brain Res. 1283, 177–185 (2009).
Walker, J. E. & Margolin, S. B. Pirfenidone for chronic progressive multiple sclerosis. Mult. Scler. 7, 305–312 (2001).
Walker, J. E., Giri, S. N. & Margolin, S. B. A double-blind, randomized, controlled study of oral pirfenidone for treatment of secondary progressive multiple sclerosis. Mult. Scler. 11, 149–158 (2005).
Chataway, J. et al. A novel adaptive design strategy increases the efficiency of clinical trials in secondary progressive multiple sclerosis. Mult. Scler. 17, 81–88 (2011).
Friede, T. et al. Designing a seamless phase II/III clinical trial using early outcomes for treatment selection: an application in multiple sclerosis. Stat. Med. 30, 1528–1540 (2011).
Simon, R. Optimal two-stage designs for phase II clinical trials. Control. Clin. Trials. 10, 1–10 (1989).
Author information
Authors and Affiliations
Contributions
M. W. Koch researched data for the article. All authors made substantial contributions to discussion of the article content, writing of the article, and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Koch, M., Cutter, G., Stys, P. et al. Treatment trials in progressive MS—current challenges and future directions. Nat Rev Neurol 9, 496–503 (2013). https://doi.org/10.1038/nrneurol.2013.148
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2013.148
This article is cited by
-
Efficacy and safety of N-acetyl-l-leucine in Niemann–Pick disease type C
Journal of Neurology (2022)
-
The timed 25-foot walk is a more sensitive outcome measure than the EDSS for PPMS trials: an analysis of the PROMISE clinical trial dataset
Journal of Neurology (2022)
-
Reaching an evidence-based prognosis for personalized treatment of multiple sclerosis
Nature Reviews Neurology (2019)
-
Siponimod pharmacokinetics, safety, and tolerability in combination with the potent CYP3A4 inhibitor itraconazole in healthy subjects with different CYP2C9 genotypes
European Journal of Clinical Pharmacology (2019)
-
Teriflunomide attenuates neuroinflammation-related neural damage in mice carrying human PLP1 mutations
Journal of Neuroinflammation (2018)