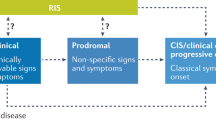
Abstract
Multiple sclerosis (MS) is an immunological disease that causes acute inflammatory lesions and chronic inflammation in the CNS, leading to tissue damage and disability. As awareness of MS has increased and options for therapy have come into use, a large amount of epidemiological data have been collected, enabling studies of changes in incidence and disease course over time. Overall, these data seem to indicate that the incidence of MS has increased, but the course of the disease has become milder, particularly in the 25 years since the first disease-modifying therapies (DMTs) became available. A clear understanding of these trends and the reasons for them is important for understanding the factors that influence the development and progression of MS, and for clinical management with respect to prevention and treatment decisions. In this Review, we consider the evidence for changes in the epidemiology of MS, focusing on trends in the incidence of the disease over time and trends in the disease severity. In addition, we discuss the factors influencing these trends, including refinement of diagnostic criteria and improvements in health-care systems that have increased diagnosis in people with mild disease, and the introduction and improvement of DMT.
Key points
-
Most studies of multiple sclerosis (MS) in the same population over time have shown that the incidence has increased.
-
At least a part of the increase in incidence can be attributed to improved public awareness, better health care, more MS specialists and MRI scanners, and changing diagnostic criteria.
-
In parallel with the increase in incidence, the disease course of MS has also changed; time to disability has lengthened and survival has improved in patients with relapsing–remitting MS.
-
Changes in disease course are likely to have resulted from more complete diagnosis of benign MS, disease-modifying therapies and lifestyle-driven changes in the natural history of the disease.
-
Understanding the reasons for the changing epidemiology of MS is important and provides insight into factors that influence development and progression of MS and for clinical management.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Alonso, A. & Hernan, M. A. Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology 71, 129–135 (2008).
Koch-Henriksen, N. & Sorensen, P. S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 9, 520–532 (2010).
Beiki, O., Frumento, P., Bottai, M., Manouchehrinia, A. & Hillert, J. Changes in the risk of reaching multiple sclerosis disability milestones in recent decades: a nationwide population-based cohort study in Sweden. JAMA Neurol. 76, 665–671 (2019).
Celius, E. G. & Vandvik, B. Multiple sclerosis in Oslo, Norway: prevalence on 1 January 1995 and incidence over a 25-year period. Eur. J. Neurol. 8, 463–469 (2001).
Simonsen, C. S., Edland, A., Berg-Hansen, P. & Celius, E. G. High prevalence and increasing incidence of multiple sclerosis in the Norwegian county of Buskerud. Acta Neurol. Scand. 135, 412–418 (2017).
Dahl, O. P., Aarseth, J. H., Myhr, K. M., Nyland, H. & Midgard, R. Multiple sclerosis in Nord-Trondelag County, Norway: a prevalence and incidence study. Acta Neurol. Scand. 109, 378–384 (2004).
Grytten, N. et al. A 50-year follow-up of the incidence of multiple sclerosis in Hordaland County, Norway. Neurology 66, 182–186 (2006).
Benjaminsen, E., Olavsen, J., Karlberg, M. & Alstadhaug, K. B. Multiple sclerosis in the far north–incidence and prevalence in Nordland County, Norway, 1970-2010. BMC Neurol. 14, 226 (2014).
Sumelahti, M. L., Holmberg, M. H., Murtonen, A., Huhtala, H. & Elovaara, I. Increasing incidence in relapsing–remitting MS and high rates among young women in Finland: a thirty-year follow-up. Mult. Scler. Int. 2014, 186950 (2014).
Debouverie, M., Pittion-Vouyovitch, S., Louis, S., Roederer, T. & Guillemin, F. Increasing incidence of multiple sclerosis among women in Lorraine, Eastern France. Mult. Scler. 13, 962–967 (2007).
Grassivaro, F. et al. Multiple sclerosis incidence and prevalence trends in the province of Padua, Northeast Italy, 1965-2018. Neuroepidemiology 52, 41–46 (2018).
Papathanasopoulos, P., Gourzoulidou, E., Messinis, L., Georgiou, V. & Leotsinidis, M. Prevalence and incidence of multiple sclerosis in western Greece: a 23-year survey. Neuroepidemiology 30, 167–173 (2008).
Ribbons, K., Lea, R., Tiedeman, C., Mackenzie, L. & Lechner-Scott, J. Ongoing increase in incidence and prevalence of multiple sclerosis in Newcastle, Australia: a 50-year study. Mult. Scler. 23, 1063–1071 (2017).
Warren, S. A., Svenson, L. W. & Warren, K. G. Contribution of incidence to increasing prevalence of multiple sclerosis in Alberta, Canada. Mult. Scler. 14, 872–879 (2008).
Alshubaili, A. F., Alramzy, K., Ayyad, Y. M. & Gerish, Y. Epidemiology of multiple sclerosis in Kuwait: new trends in incidence and prevalence. Eur. Neurol. 53, 125–131 (2005).
Saadatnia, M., Etemadifar, M. & Maghzi, A. H. Multiple sclerosis in Isfahan, Iran. Int. Rev. Neurobiol. 79, 357–375 (2007).
Elhami, S. R., Mohammad, K., Sahraian, M. A. & Eftekhar, H. A 20-year incidence trend (1989-2008) and point prevalence (March 20, 2009) of multiple sclerosis in Tehran, Iran: a population-based study. Neuroepidemiology 36, 141–147 (2011).
Etemadifar, M. & Maghzi, A. H. Sharp increase in the incidence and prevalence of multiple sclerosis in Isfahan, Iran. Mult. Scler. 17, 1022–1027 (2011).
Etemadifar, M. et al. Estimated prevalence and incidence of multiple sclerosis in Iran. Eur. Neurol. 72, 370–374 (2014).
Eskandarieh, S., Heydarpour, P., Elhami, S. R. & Sahraian, M. A. Prevalence and incidence of multiple sclerosis in Tehran, Iran. Iran J. Public Heal. 46, 699–704 (2017).
Cheraghmakani, H., Baghbanian, S. M., HabibiSaravi, R., Azar, A. & Ghasemihamedani, F. Age and sex-adjusted incidence and yearly prevalence of multiple sclerosis (MS) in Mazandaran province, Iran: an 11-years study. PLoS ONE 15, e0235562 (2020).
Etemadifar, M., Nikanpour, Y., Neshatfar, A., Mansourian, M. & Fitzgerald, S. Incidence and prevalence of multiple sclerosis in Persian Gulf area: a systematic review and meta-analysis. Mult. Scler. Relat. Disord. 40, 101959 (2020).
Willumsen, J. S., Aarseth, J. H., Myhr, K. M. & Midgard, R. High incidence and prevalence of MS in Møre and Romsdal County, Norway, 1950-2018. Neurol. Neuroimmunol. Neuroinflamm. 7, e713 (2020).
Koch-Henriksen, N., Thygesen, L. C., Stenager, E., Laursen, B. & Magyari, M. Incidence of MS has increased markedly over six decades in Denmark particularly with late onset and in women. Neurology 90, e1954–e1963 (2018).
Sumelahti, M. L., Tienari, P. J., Hakama, M. & Wikstrom, J. Multiple sclerosis in Finland: incidence trends and differences in relapsing remitting and primary progressive disease courses. J. Neurol. Neurosurg. Psychiatry 74, 25–28 (2003).
Westerlind, H., Stawiarz, L., Fink, K., Hillert, J. & Manouchehrinia, A. A significant decrease in diagnosis of primary progressive multiple sclerosis: a cohort study. Mult. Scler. 22, 1071–1079 (2016).
Ahlgren, C., Oden, A. & Lycke, J. High nationwide incidence of multiple sclerosis in Sweden. PLoS ONE 9, e108599 (2014).
Kingwell, E. et al. High incidence and increasing prevalence of multiple sclerosis in British Columbia, Canada: findings from over two decades (1991-2010). J. Neurol. 262, 2352–2363 (2015).
Mayr, W. T. et al. Incidence and prevalence of multiple sclerosis in Olmsted County, Minnesota, 1985-2000. Neurology 61, 1373–1377 (2003).
Daltrozzo, T., Hapfelmeier, A., Donnachie, E., Schneider, A. & Hemmer, B. A systematic assessment of prevalence, incidence and regional distribution of multiple sclerosis in Bavaria from 2006 to 2015. Front. Neurol. 9, 871 (2018).
Hader, W. J. & Yee, I. M. Incidence and prevalence of multiple sclerosis in Saskatoon, Saskatchewan. Neurology 69, 1224–1229 (2007).
Rotstein, D. L. et al. Temporal trends in multiple sclerosis prevalence and incidence in a large population. Neurology 90, e1435–e1441 (2018).
Zamboni, P. et al. A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency. J. Vasc. Surg. 50, 1348–1358 (2009).
Iljicsov, A. et al. Incidence and prevalence of multiple sclerosis in Hungary based on record linkage of nationwide multiple healthcare administrative data. PLoS ONE 15, e0236432 (2020).
Grytten, N., Aarseth, J. H., Lunde, H. M. & Myhr, K. M. A 60-year follow-up of the incidence and prevalence of multiple sclerosis in Hordaland County, Western Norway. J. Neurol. Neurosurg. Psychiatry 87, 100–105 (2016).
Marrie, R. A. et al. The incidence and prevalence of multiple sclerosis in Nova Scotia, Canada. Can. J. Neurol. Sci. 40, 824–831 (2013).
Widdifield, J. et al. Development and validation of an administrative data algorithm to estimate the disease burden and epidemiology of multiple sclerosis in Ontario, Canada. Mult. Scler. 21, 1045–1054 (2015).
Hirst, C. et al. Increasing prevalence and incidence of multiple sclerosis in South East Wales. J. Neurol. Neurosurg. Psychiatry 80, 386–391 (2009).
Kotzamani, D. et al. Rising incidence of multiple sclerosis in females associated with urbanization. Neurology 78, 1728–1735 (2012).
Trojano, M. et al. Geographical variations in sex ratio trends over time in multiple sclerosis. PLoS ONE 7, e48078 (2012).
Valadkeviciene, D., Kavaliunas, A., Kizlaitiene, R., Jocys, M. & Jatuzis, D. Incidence rate and sex ratio in multiple sclerosis in Lithuania. Brain Behav. 9, e01150 (2019).
Ramagopalan, S. V. et al. Sex ratio of multiple sclerosis and clinical phenotype. Eur. J. Neurol. 17, 634–637 (2010).
Kampman, M. T. et al. Sex ratio of multiple sclerosis in persons born from 1930 to 1979 and its relation to latitude in Norway. J. Neurol. 260, 1481–1488 (2013).
Brain, W. R. Critical review: disseminated sclerosis. Q. J. Med 23, 343–391 (1930).
Tremlett, H. Slower MS disability progression than previously reported. ECTRIMS https://onlinelibrary.ectrims-congress.eu/ectrims/2019/stockholm/279360/helen.tremlett.slower.ms.disability.progression.than.previously.reported.html (2019).
Kalincik, T. et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain 136, 3609–3617 (2013).
Tintore, M. et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain 138, 1863–1874 (2015).
Hernan, M. A., Olek, M. J. & Ascherio, A. Cigarette smoking and incidence of multiple sclerosis. Am. J. Epidemiol. 154, 69–74 (2001).
Riise, T., Nortvedt, M. W. & Ascherio, A. Smoking is a risk factor for multiple sclerosis. Neurology 61, 1122–1124 (2003).
Hawkes, C. H. Smoking is a risk factor for multiple sclerosis: a metanalysis. Mult. Scler. 13, 610–615 (2007).
Munger, K. L. et al. Childhood body mass index and multiple sclerosis risk: a long-term cohort study. Mult. Scler. 19, 1323–1329 (2013).
Nielsen, N. M. et al. Reproductive history and risk of multiple sclerosis. Epidemiology 22, 546–552 (2011).
Allison, R. A. & Miller, J. D. H. Prevalence and familial incidence of disseminated sclerosis. Ulster. Med. J. 23 (Suppl. 2), 5–27 (1954).
Schumacher, G. et al. Problems of experimental trials of therapy in multiple sclerosis: report by the Panel on the Evaluation of Experimental Trials of Therapy in Multiple Sclerosis. Ann. NY Acad. Sci. 122, 552–568 (1965).
Poser, C. M. et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann. Neurol. 13, 227–231 (1983).
McDonald, W. I. et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann. Neurol. 50, 121–127 (2001).
Polman, C. H. et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘McDonald criteria’. Ann. Neurol. 58, 840–846 (2005).
Polman, C. H. et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 69, 292–302 (2011).
Thompson, A. J. et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 17, 162–173 (2018).
McNicholas, N., Hutchinson, M., McGuigan, C. & Chataway, J. 2017 McDonald diagnostic criteria: a review of the evidence. Mult. Scler. Relat. Disord. 24, 48–54 (2018).
van der Vuurst de Vries, R. M. et al. Application of the 2017 revised McDonald criteria for multiple sclerosis to patients with a typical clinically isolated syndrome. JAMA Neurol. 75, 1392–1398 (2018).
Gobbin, F. et al. 2017 McDonald criteria for multiple sclerosis: earlier diagnosis with reduced specificity? Mult. Scler. Relat. Disord. 29, 23–25 (2019).
Gaetani, L. et al. 2017 revisions of McDonald criteria shorten the time to diagnosis of multiple sclerosis in clinically isolated syndromes. J. Neurol. 265, 2684–2687 (2018).
Miller, D. H., Chard, D. T. & Ciccarelli, O. Clinically isolated syndromes. Lancet Neurol. 11, 157–169 (2012).
Novakova, L. et al. Clinically isolated syndromes with no further disease activity suggestive of multiple sclerosis at the age of population life expectancy. Mult. Scler. 20, 496–500 (2014).
Kerbrat, A. et al. Ten-year prognosis in multiple sclerosis: a better outcome in relapsing-remitting patients but not in primary progressive patients. Eur. J. Neurol. 22, 507–e35 (2015).
Kuhle, J. et al. Conversion from clinically isolated syndrome to multiple sclerosis: a large multicentre study. Mult. Scler. 21, 1013–1024 (2015).
Tintore, M. et al. The long-term outcomes of CIS patients in the Barcelona inception cohort: looking back to recognize aggressive MS. Mult. Scler. 26, 1658–1669 (2019).
Adornato, B. T. et al. The practice of neurology, 2000-2010: report of the AAN Member Research Subcommittee. Neurology 77, 1921–1928 (2011).
Canadian Agency for Drugs and Technologies in Health. In Brief: Canadian medical imaging inventory: 2017 (CADTH, 2018).
Michas, F. Total number of magnetic resonance imaging (MRI) units in the United Kingdom (UK) from 2000 to 2014. Statista https://www.statista.com/statistics/473302/number-of-magnetic-resonance-imaging-units-united-kingdom-uk/ (2019).
Gianfrancesco, M. A. et al. Obesity during childhood and adolescence increases susceptibility to multiple sclerosis after accounting for established genetic and environmental risk factors. Obes. Res. Clin. Pract. 8, e435–e447 (2014).
Stamatakis, E., Primatesta, P., Chinn, S., Rona, R. & Falascheti, E. Overweight and obesity trends from 1974 to 2003 in English children: what is the role of socioeconomic factors? Arch. Dis. Child. 90, 999–1004 (2005).
Hedstrom, A. K., Baarnhielm, M., Olsson, T. & Alfredsson, L. Tobacco smoking, but not Swedish snuff use, increases the risk of multiple sclerosis. Neurology 73, 696–701 (2009).
Ramagopalan, S. V. et al. Association of smoking with risk of multiple sclerosis: a population-based study. J. Neurol. 260, 1778–1781 (2013).
Thun, M. J., Henley, S. J. & Calle, E. E. Tobacco use and cancer: an epidemiologic perspective for genetics. Oncogene 21, 7307–7325 (2002).
Hedstrom, A. K., Baarnhielm, M., Olsson, T. & Alfredsson, L. Exposure to environmental tobacco smoke is associated with increased risk for multiple sclerosis. Mult. Scler. 17, 788–793 (2011).
Oturai, D. B. et al. Exposure to passive smoking during adolescence is associated with an increased risk of developing multiple sclerosis. Mult. Scler. J. 27, 188–197 (2021).
Magyari, M., Koch-Henriksen, N., Pfleger, C. C. & Sorensen, P. S. Reproduction and the risk of multiple sclerosis. Mult. Scler. 19, 1604–1609 (2013).
Goldacre, M. J., Wotton, C. J., Seagroatt, V. & Yeates, D. Multiple sclerosis after infectious mononucleosis: record linkage study. J. Epidemiol. Community Heal. 58, 1032–1035 (2004).
Nielsen, T. R. et al. Multiple sclerosis after infectious mononucleosis. Arch. Neurol. 64, 72–75 (2007).
Kuri, A. et al. Epidemiology of Epstein-Barr virus infection and infectious mononucleosis in the United Kingdom. BMC Public Health 20, 912 (2020).
Bergamaschi, R. et al. PM(2.5) exposure as a risk factor for multiple sclerosis. An ecological study with a Bayesian mapping approach. Environ. Sci. Pollut. Res. Int. 28, 2804–2809 (2021).
Bihrmann, K. et al. Small-scale geographical variation in multiple sclerosis: a case-control study using Danish register data 1971-2013. Mult. Scler. Relat. Disord. 23, 40–45 (2018).
Kurtzke, J. F. Disability rating scales in multiple sclerosis. Ann. NY Acad. Sci. 436, 347–360 (1984).
Weinshenker, B. G. et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 112, 133–146 (1989).
Runmarker, B. & Andersen, O. Prognostic factors in a multiple sclerosis incidence cohort with twenty-five years of follow-up. Brain 116, 117–134 (1993).
Myhr, K. M. et al. Disability and prognosis in multiple sclerosis: demographic and clinical variables important for the ability to walk and awarding of disability pension. Mult. Scler. 7, 59–65 (2001).
Confavreux, C. Establishment and use of multiple sclerosis registers–EDMUS. Ann. Neurol. 36, S136–S139 (1994).
Confavreux, C., Vukusic, S., Moreau, T. & Adeleine, P. Relapses and progression of disability in multiple sclerosis. N. Engl. J. Med. 343, 1430–1438 (2000).
Confavreux, C., Vukusic, S. & Adeleine, P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain 126, 770–782 (2003).
Confavreux, C. & Vukusic, S. Age at disability milestones in multiple sclerosis. Brain 129, 595–605 (2006).
Leray, E. et al. Evidence for a two-stage disability progression in multiple sclerosis. Brain 133, 1900–1913 (2010).
Tremlett, H., Paty, D. & Devonshire, V. Disability progression in multiple sclerosis is slower than previously reported. Neurology 66, 172–177 (2006).
Veugelers, P. J. et al. Disease progression among multiple sclerosis patients before and during a disease-modifying drug program: a longitudinal population-based evaluation. Mult. Scler. 15, 1286–1294 (2009).
Hillert, J. & Stawiarz, L. The Swedish MS registry–clinical support tool and scientific resource. Acta Neurol. Scand. 132, 11–19 (2015).
Manouchehrinia, A., Beiki, O. & Hillert, J. Clinical course of multiple sclerosis: a nationwide cohort study. Mult. Scler. 23, 1488–1495 (2017).
Damasceno, A., von, G. F., Brandao, C. O., Damasceno, B. P. & Cendes, F. Prognostic indicators for long-term disability in multiple sclerosis patients. J. Neurol. Sci. 324, 29–33 (2013).
Capra, R. et al. Assessing long-term prognosis improvement as a consequence of treatment pattern changes in MS. Mult. Scler. 23, 1757–1761 (2017).
Kister, I. et al. Increasing age at disability milestones among MS patients in the MSBase registry. J. Neurol. Sci. 318, 94–99 (2012).
Shirani, A., Zhao, Y., Kingwell, E., Rieckmann, P. & Tremlett, H. Temporal trends of disability progression in multiple sclerosis: findings from British Columbia, Canada (1975-2009). Mult. Scler. 18, 442–450 (2012).
Koch, M., Kingwell, E., Rieckmann, P. & Tremlett, H. The natural history of secondary progressive multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 81, 1039–1043 (2010).
Cottrell, D. A. et al. The natural history of multiple sclerosis: a geographically based study. 5. The clinical features and natural history of primary progressive multiple sclerosis. Brain 122, 625–639 (1999).
Tremlett, H., Paty, D. & Devonshire, V. The natural history of primary progressive MS in British Columbia, Canada. Neurology 65, 1919–1923 (2005).
Leray, E. et al. Long-term survival of patients with multiple sclerosis in West France. Mult. Scler. 13, 865–874 (2007).
Smestad, C., Sandvik, L. & Celius, E. G. Excess mortality and cause of death in a cohort of Norwegian multiple sclerosis patients. Mult. Scler. 15, 1263–1270 (2009).
Capkun, G. et al. Mortality and comorbidities in patients with multiple sclerosis compared with a population without multiple sclerosis: an observational study using the US Department of Defense administrative claims database. Mult. Scler. Relat. Disord. 4, 546–554 (2015).
Ragonese, P., Aridon, P., Salemi, G., D’Amelio, M. & Savettieri, G. Mortality in multiple sclerosis: a review. Eur. J. Neurol. 15, 123–127 (2008).
Manouchehrinia, A., Tanasescu, R., Tench, C. R. & Constantinescu, C. S. Mortality in multiple sclerosis: meta-analysis of standardised mortality ratios. J. Neurol. Neurosurg. Psychiatry 87, 324–331 (2016).
Kingwell, E. et al. Relative mortality and survival in multiple sclerosis: findings from British Columbia, Canada. J. Neurol. Neurosurg. Psychiatry 83, 61–66 (2012).
Lunde, H. M. B., Assmus, J., Myhr, K. M., Bo, L. & Grytten, N. Survival and cause of death in multiple sclerosis: a 60-year longitudinal population study. J. Neurol. Neurosurg. Psychiatry 88, 621–625 (2017).
Koch-Henriksen, N., Laursen, B., Stenager, E. & Magyari, M. Excess mortality among patients with multiple sclerosis in Denmark has dropped significantly over the past six decades: a population based study. J. Neurol. Neurosurg. Psychiatry 88, 626–631 (2017).
Burkill, S. et al. Mortality trends for multiple sclerosis patients in Sweden from 1968 to 2012. Neurology 89, 555–562 (2017).
Ramsaransing, G., Maurits, N., Zwanikken, C. & De, K. J. Early prediction of a benign course of multiple sclerosis on clinical grounds: a systematic review. Mult. Scler. 7, 345–347 (2001).
Amato, M. P. et al. Benign multiple sclerosis: cognitive, psychological and social aspects in a clinical cohort. J. Neurol. 253, 1054–1059 (2006).
Glad, S., Nyland, H. & Myhr, K. M. Benign multiple sclerosis. Acta Neurol. Scand. Suppl. 183, 55–57 (2006).
Gajofatto, A. et al. Benign multiple sclerosis: physical and cognitive impairment follow distinct evolutions. Acta Neurol. Scand. 133, 183–191 (2016).
Sayao, A. L., Devonshire, V. & Tremlett, H. Longitudinal follow-up of ‘benign’ multiple sclerosis at 20 years. Neurology 68, 496–500 (2007).
Hirst, C. et al. Change in disability in patients with multiple sclerosis: a 20-year prospective population-based analysis. J. Neurol. Neurosurg. Psychiatry 79, 1137–1143 (2008).
Tallantyre, E. C. et al. How common is truly benign MS in a UK population? J. Neurol. Neurosurg. Psychiatry 90, 522–528 (2019).
Skoog, B., Runmarker, B., Winblad, S., Ekholm, S. & Andersen, O. A representative cohort of patients with non-progressive multiple sclerosis at the age of normal life expectancy. Brain 135, 900–911 (2012).
Pfleger, C. C., Flachs, E. M. & Koch-Henriksen, N. Social consequences of multiple sclerosis (1): Early pension and temporary unemployment–a historical prospective cohort study. Mult. Scler. 16, 121–126 (2010).
Ellenberger, D. et al. Is benign MS really benign? What a meaningful classification beyond the EDSS must take into consideration. Mult. Scler. Relat. Disord. 46, 102485 (2020).
Casserly, C. & Ebers, G. C. Relapses do not matter in relation to long-term disability: yes. Mult. Scler. 17, 1412–1414 (2011).
Lublin, F. D. Relapses do not matter in relation to long-term disability: no (they do). Mult. Scler. 17, 1415–1416 (2011).
Hutchinson, M. Relapses do not matter in relation to long-term disability: commentary. Mult. Scler. 17, 1417 (2011).
Cree, B. A. C. et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann. Neurol. 85, 653–666 (2019).
Koch-Henriksen, N., Thygesen, L. C., Sorensen, P. S. & Magyari, M. Worsening of disability caused by relapses in multiple sclerosis: a different approach. Mult. Scler. Relat. Disord. 32, 1–8 (2019).
Tedeholm, H. et al. Time to secondary progression in patients with multiple sclerosis who were treated with first generation immunomodulating drugs. Mult. Scler. 19, 765–774 (2013).
Palace, J. et al. Assessing the long-term effectiveness of interferon-beta and glatiramer acetate in multiple sclerosis: final 10-year results from the UK multiple sclerosis risk-sharing scheme. J. Neurol. Neurosurg. Psychiatry 90, 251–260 (2018).
Tremlett, H., Zhao, Y., Rieckmann, P. & Hutchinson, M. New perspectives in the natural history of multiple sclerosis. Neurology 74, 2004–2015 (2010).
Trojano, M. et al. New natural history of interferon-β-treated relapsing multiple sclerosis. Ann. Neurol. 61, 300–306 (2007).
Trojano, M. et al. Real-life impact of early interferonβ therapy in relapsing multiple sclerosis. Ann. Neurol. 66, 513–520 (2009).
Chalmer, T. A. et al. Early versus later treatment start in multiple sclerosis: a register-based cohort study. Eur. J. Neurol. 25, 1262.e110 (2018).
The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 43, 655–661 (1993).
Goodin, D. S. et al. Survival in MS: a randomized cohort study 21 years after the start of the pivotal IFNβ-1b trial. Neurology 78, 1315–1322 (2012).
Amato, M. P. et al. Disease-modifying drugs can reduce disability progression in relapsing multiple sclerosis. Brain 143, 3013–3024 (2020).
Kalincik, T. et al. Effect of disease-modifying therapy on disability in relapsing-remitting multiple sclerosis over 15 years. Neurology 96, e783–e797 (2021).
Kingwell, E. et al. Assessment of cancer risk with β-interferon treatment for multiple sclerosis. J. Neurol. Neurosurg.Psychiatry 85, 1096–1102 (2014).
Sormani, M. P. et al. Will Rogers phenomenon in multiple sclerosis. Ann. Neurol. 64, 428–433 (2008).
Sormani, M. P. The Will Rogers phenomenon: the effect of different diagnostic criteria. J. Neurol. Sci. 287 (Suppl. 1), 46–49 (2009).
Tintore, M. et al. New diagnostic criteria for multiple sclerosis: application in first demyelinating episode. Neurology 60, 27–30 (2003).
Handel, A. E. et al. Smoking and multiple sclerosis: an updated meta-analysis. PLoS ONE 6, e16149 (2011).
Degelman, M. L. & Herman, K. M. Smoking and multiple sclerosis: a systematic review and meta-analysis using the Bradford Hill criteria for causation. Mult. Scler. Relat. Disord. 17, 207–216 (2017).
Ng, M. et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA 311, 183–192 (2014).
Jakimovski, D. et al. Dietary and lifestyle factors in multiple sclerosis progression: results from a 5-year longitudinal MRI study. J. Neurol. 266, 866–875 (2019).
Atkinson, S. A. & Fleet, J. C. Canadian recommendations for vitamin D intake for persons affected by multiple sclerosis. J. Steroid Biochem. 199, 105606 (2020).
Karim, M. E., Gustafson, P., Petkau, J. & Tremlett, H. Comparison of statistical approaches for dealing with immortal time bias in drug effectiveness studies. Am. J. Epidemiol. 184, 325–335 (2016).
Cain, K. C. et al. Bias due to left truncation and left censoring in longitudinal studies of developmental and disease processes. Am. J. Epidemiol. 173, 1078–1084 (2011).
McKay, K. A. et al. A population-based study comparing multiple sclerosis clinic users and non-users in British Columbia, Canada. Eur. J. Neurol. 23, 1093–1100 (2016).
Author information
Authors and Affiliations
Contributions
N.K.-H. researched data for the article and wrote the article. Both authors made substantial contributions to discussion of the content and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
In the past 2 years, N.K.-H. has received support for participation in congresses and symposia from Sanofi Genzyme. M.M. has served on scientific advisory boards for Abbvie, Biogen, Merck, Novartis, Roche, Sanofi and Teva, has received honoraria for lecturing from Biogen, Genzyme, Merck, Novartis and Sanofi, support for congress participation from Biogen, Genzyme, Roche and Teva, and research grants from Merck, Novartis and Sanofi.
Additional information
Peer review information
Nature Reviews Neurology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koch-Henriksen, N., Magyari, M. Apparent changes in the epidemiology and severity of multiple sclerosis. Nat Rev Neurol 17, 676–688 (2021). https://doi.org/10.1038/s41582-021-00556-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-021-00556-y
This article is cited by
-
Cost and effectiveness of autologous haematopoietic stem cell transplantation and high-efficacy disease-modifying therapies in relapsing–remitting multiple sclerosis
Neurological Sciences (2024)
-
“So at least now I know how to deal with things myself, what I can do if it gets really bad again”—experiences with a long-term cross-sectoral advocacy care and case management for severe multiple sclerosis: a qualitative study
BMC Health Services Research (2024)
-
Association between SUMF1 polymorphisms and COVID-19 severity
BMC Genomic Data (2023)
-
The application of Aptamer in biomarker discovery
Biomarker Research (2023)
-
Impact of the first Gulf war on multiple sclerosis risk in Kuwait: a quasi-experimental study
BMC Neurology (2023)