Key Points
-
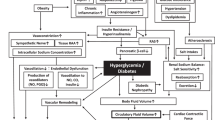
In addition to classical insulin target tissues (liver, skeletal muscle and white adipose tissue) insulin acts on most human organs and cell types, including the arterial vasculature and the kidney
-
In insulin-resistant states such as obesity or type 2 diabetes mellitus, not only are the classical insulin effects impaired, but also the effects of insulin on the vasculature and the kidney
-
Insulin stimulates its own delivery to target cells by actions on the vasculature involving increased capillary recruitment and endothelial transcytosis; these effects are impaired in insulin-resistant states
-
Insulin resistance affects many aspects of kidney function, including renal haemodynamics, podocyte viability and tubular function
-
The action of insulin on renal sodium handling is preserved in insulin resistance and contributes to sodium retention and arterial hypertension
-
Renal and vascular insulin resistance can be improved through an integrated approach including lifestyle interventions and pharmacological agents
Abstract
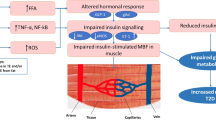
Insulin resistance is a systemic disorder that affects many organs and insulin-regulated pathways. The disorder is characterized by a reduced action of insulin despite increased insulin concentrations (hyperinsulinaemia). The effects of insulin on the kidney and vasculature differ in part from the effects on classical insulin target organs. Insulin causes vasodilation by enhancing endothelial nitric oxide production through activation of the phosphatidylinositol 3-kinase pathway. In insulin-resistant states, this pathway is impaired and the mitogen-activated protein kinase pathway stimulates vasoconstriction. The action of insulin on perivascular fat tissue and the subsequent effects on the vascular wall are not fully understood, but the hepatokine fetuin-A, which is released by fatty liver, might promote the proinflammatory effects of perivascular fat. The strong association of salt-sensitive arterial hypertension with insulin resistance indicates an involvement of the kidney in the insulin resistance syndrome. The insulin receptor is expressed on renal tubular cells and podocytes and insulin signalling has important roles in podocyte viability and tubular function. Renal sodium transport is preserved in insulin resistance and contributes to the salt-sensitivity of blood pressure in hyperinsulinaemia. Therapeutically, renal and vascular insulin resistance can be improved by an integrated holistic approach aimed at restoring overall insulin sensitivity and improving insulin signalling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Cuatrecasas, P. The insulin receptor. Diabetes 21, 396–402 (1972).
Olefsky, J. M. Insulin binding, biologic activity, and metabolism of biosynthetic human insulin. Diabetes Care 4, 244–247 (1981).
Kahn, C. R., Neville, D. M. Jr & Roth, J. Insulin-receptor interaction in the obese-hyperglycemic mouse. A model of insulin resistance. J. Biol. Chem. 248, 244–250 (1973).
Groop, L. C. et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J. Clin. Invest. 84, 205–213 (1989).
Prager, R., Wallace, P. & Olefsky, J. M. In vivo kinetics of insulin action on peripheral glucose disposal and hepatic glucose output in normal and obese subjects. J. Clin. Invest. 78, 472–481 (1986).
Rask-Madsen, C. & Kahn, C. R. Tissue-specific insulin signaling, metabolic syndrome, and cardiovascular disease. Arterioscler Thromb. Vasc. Biol. 32, 2052–2059 (2012).
Heni, M., Kullmann, S., Preissl, H., Fritsche, A. & Haring, H. U. Impaired insulin action in the human brain: causes and metabolic consequences. Nat. Rev. Endocrinol. 11, 701–711 (2015). This review summarizes the current knowledge of normal and impaired cerebral insulin effects.
Kellerer, M. et al. Distinct alpha-subunit structures of human insulin receptor A and B variants determine differences in tyrosine kinase activities. Biochemistry 31, 4588–4596 (1992).
Seino, S. & Bell, G. I. Alternative splicing of human insulin receptor messenger RNA. Biochem. Biophys. Res. Commun. 159, 312–316 (1989).
Belfiore, A., Frasca, F., Pandini, G., Sciacca, L. & Vigneri, R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev. 30, 586–623 (2009).
Kasuga, M., Karlsson, F. A. & Kahn, C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science 215, 185–187 (1982).
Backer, J. M. et al. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 11, 3469–3479 (1992).
Sun, X. J. et al. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature 352, 73–77 (1991).
Taniguchi, C. M., Emanuelli, B. & Kahn, C. R. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7, 85–96 (2006).
Farese, R. V., Sajan, M. P. & Standaert, M. L. Atypical protein kinase C in insulin action and insulin resistance. Biochem. Soc. Trans. 33, 350–353 (2005).
Manning, B. D. & Cantley, L. C. AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 (2007).
Brady, M. J. & Saltiel, A. R. The role of protein phosphatase-1 in insulin action. Recent Prog. Horm. Res. 56, 157–173 (2001).
Elchebly, M. et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283, 1544–1548 (1999).
Lazar, D. F. & Saltiel, A. R. Lipid phosphatases as drug discovery targets for type 2 diabetes. Nat. Rev. Drug Discov. 5, 333–342 (2006).
Vinciguerra, M. & Foti, M. PTEN and SHIP2 phosphoinositide phosphatases as negative regulators of insulin signalling. Arch. Physiol. Biochem. 112, 89–104 (2006).
Emanuelli, B. et al. SOCS-3 is an insulin-induced negative regulator of insulin signaling. J. Biol. Chem. 275, 15985–15991 (2000).
Holt, L. J. & Siddle, K. Grb10 and Grb14: enigmatic regulators of insulin action—and more? Biochem. J. 388, 393–406 (2005).
Copps, K. D. & White, M. F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55, 2565–2582 (2012).
Fritsche, L. et al. Insulin-induced serine phosphorylation of IRS-2 via ERK1/2 and mTOR: studies on the function of Ser675 and Ser907. Am. J. Physiol. Endocrinol. Metab. 300, E824–836 (2011).
Neukamm, S. S. et al. Phosphorylation of serine 1137/1138 of mouse insulin receptor substrate (IRS) 2 regulates cAMP-dependent binding to 14-3-3 proteins and IRS2 protein degradation. J. Biol. Chem. 288, 16403–16415 (2013).
Weigert, C. et al. Interplay and effects of temporal changes in the phosphorylation state of serine-302, -307, and -318 of insulin receptor substrate-1 on insulin action in skeletal muscle cells. Mol. Endocrinol. 22, 2729–2740 (2008).
Weigert, C. et al. The phosphorylation of Ser318 of insulin receptor substrate 1 is not per se inhibitory in skeletal muscle cells but is necessary to trigger the attenuation of the insulin-stimulated signal. J. Biol. Chem. 280, 37393–37399 (2005). This study shows the complex molecular regulation of the function of insulin receptor substrate 1 by specific serine phosphorylation.
Boucher, J., Kleinridders, A. & Kahn, C. R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol 6 a009191 (2014).
Kowluru, A. & Matti, A. Hyperactivation of protein phosphatase 2A in models of glucolipotoxicity and diabetes: potential mechanisms and functional consequences. Biochem. Pharmacol. 84, 591–597 (2012).
Vaidyanathan, K. & Wells, L. Multiple tissue-specific roles for the O-GlcNAc post-translational modification in the induction of and complications arising from type II diabetes. J. Biol. Chem. 289, 34466–34471 (2014).
Potenza, M. A., Addabbo, F. & Montagnani, M. Vascular actions of insulin with implications for endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 297, E568–577 (2009).
Hale, L. J. & Coward, R. J. The insulin receptor and the kidney. Curr. Opin. Nephrol. Hypertens. 22, 100–106 (2013).
Coward, R. J. et al. The human glomerular podocyte is a novel target for insulin action. Diabetes 54, 3095–3102 (2005).
Conti, F. G. et al. Studies on binding and mitogenic effect of insulin and insulin-like growth factor I in glomerular mesangial cells. Endocrinology 122, 2788–2795 (1988).
Conti, F. G., Elliot, S. J., Striker, L. J. & Striker, G. E. Binding of insulin-like growth factor-I by glomerular endothelial and epithelial cells: further evidence for IGF-I action in the renal glomerulus. Biochem. Biophys. Res. Commun. 163, 952–958 (1989).
Nakamura, R., Emmanouel, D. S. & Katz, A. I. Insulin binding sites in various segments of the rabbit nephron. J. Clin. Invest. 72, 388–392 (1983).
Ejerblad, E. et al. Obesity and risk for chronicrenal failure. J. Am. Soc. Nephrol. 17, 1695–1702 (2006).
Fox, C. S. et al. Predictors of new-onset kidney disease in a community-based population. Jama 291, 844–850 (2004).
Kanasaki, K., Kitada, M., Kanasaki, M. & Koya, D. The biological consequence of obesity on the kidney. Nephrol. Dial. Transplant 28, (Suppl. 4), 1–7 (2013).
Pinto-Sietsma, S. J. et al. A central body fat distribution is related to renal function impairment, even in lean subjects. Am. J. Kidney Dis. 41, 733–741 (2003).
Ritz, E. Metabolic syndrome and kidney disease. Blood Purif. 26, 59–62 (2008).
Kramer, H. et al. Waist Circumference, Body Mass Index, and ESRD in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am. J. Kidney Dis. 67, 62–69 (2016).
Chandie Shaw, P. K. et al. Central obesity is an independent risk factor for albuminuria in nondiabetic South Asian subjects. Diabetes Care 30, 1840–1844 (2007).
Cirillo, M. et al. Microalbuminuria in nondiabetic adults: relation of blood pressure, body mass index, plasma cholesterol levels, and smoking: The Gubbio Population Study. Arch. Intern. Med. 158, 1933–1939 (1998).
Tozawa, M. et al. Influence of smoking and obesity on the development of proteinuria. Kidney Int. 62, 956–962 (2002).
Nerpin, E. et al. Insulin sensitivity measured with euglycemic clamp is independently associated with glomerular filtration rate in a community-based cohort. Diabetes Care 31, 1550–1555 (2008).
De Cosmo, S., Menzaghi, C., Prudente, S. & Trischitta, V. Role of insulin resistance in kidney dysfunction: insights into the mechanism and epidemiological evidence. Nephrol. Dial. Transplant 28, 29–36 (2013).
Saltiel, A. R. & Kahn, C. R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806 (2001).
Karlsson, F. H. et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498, 99–103 (2013).
Odegaard, J. I. & Chawla, A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 339, 172–177 (2013).
Adamczak, M. & Wiecek, A. The adipose tissue as an endocrine organ. Semin. Nephrol. 33, 2–13 (2013).
Sharma, K. et al. Adiponectin regulates albuminuria and podocyte function in mice. J. Clin. Invest. 118, 1645–1656 (2008).
Wolf, G. et al. Leptin stimulates proliferation and TGF-beta expression in renal glomerular endothelial cells: potential role in glomerulosclerosis [seecomments]. Kidney Int. 56, 860–872 (1999).
Nerlich, A. G., Schleicher, E. D., Wiest, I., Specks, U. & Timpl, R. Immunohistochemical localization of collagen VI in diabetic glomeruli. Kidney Int. 45, 1648–1656 (1994).
Stefan, N. et al. Obesity and renal disease: not all fat is created equal and not all obesity is harmful to the kidneys. Nephrol. Dial. Transplant 56, 860–872 (2014).
Stefan, N. & Haring, H. U. The role of hepatokines in metabolism. Nat. Rev. Endocrinol. 9, 144–152 (2013).
Stefan, N., Haring, H. U., Hu, F. B. & Schulze, M. B. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 1, 152–162 (2013).
Stefan, N. et al. Identification and characterization of metabolically benign obesity in humans. Arch. Intern. Med. 168, 1609–1616 (2008).
Haukeland, J. W. et al. Fetuin A in nonalcoholic fatty liver disease: in vivo and in vitro studies. Eur. J. Endocrinol. 166, 503–510 (2012).
Lehmann, R. et al. Circulating lysophosphatidylcholines are markers of a metabolically benign nonalcoholic fatty liver. Diabetes Care 36, 2331–2338 (2013).
Stefan, N. & Haring, H. U. The metabolically benign and malignant fatty liver. Diabetes 60, 2011–2017 (2011).
Stefan, N. et al. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 29, 853–857 (2006).
Auberger, P. et al. Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell 58, 631–640 (1989).
Hennige, A. M. et al. Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS ONE 3, e1765 (2008).
Ix, J. H. et al. Fetuin-A and incident diabetes mellitus in older persons. Jama 300, 182–188 (2008).
Stefan, N. et al. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes 57, 2762–2767 (2008).
Fisher, E. et al. Association of AHSG gene polymorphisms with fetuin-A plasma levels and cardiovascular diseases in the EPIC-Potsdam study. Circ. Cardiovasc. Genet. 2, 607–613 (2009).
Weikert, C. et al. Plasma fetuin-a levels and the risk of myocardial infarction and ischemic stroke. Circulation 118, 2555–2562 (2008).
Pal, D. et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 18, 1279–1285 (2012).
Stefan, N. & Haring, H. U. Circulating fetuin-A and free fatty acids interact to predict insulin resistance in humans. Nat. Med. 19, 394–395 (2013).
Stefan, N., Schick, F. & Haring, H. U. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 371, 2236–2237 (2014).
Schafer, C. et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J. Clin. Invest. 112, 357–366 (2003).
Li, M. et al. Association between higher serum fetuin-A concentrations and abnormal albuminuria in middle-aged and elderly chinese with normal glucose tolerance. Diabetes Care 33, 2462–2464 (2010).
Page, M. M. & Watkins, P. J. Provocation of postural hypotension by insulin in diabetic autonomic neuropathy. Diabetes 25, 90–95 (1976).
Baron, A. D. Hemodynamic actions of insulin. Am. J. Physiol. 267, E187–E202 (1994).
Laakso, M., Edelman, S. V., Brechtel, G. & Baron, A. D. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J. Clin. Invest. 85, 1844–1852 (1990). This study provides experimental evidence for insulin-mediated vasodilation and its increasing impairment in patients with insulin-resistance and diabetes.
Laakso, M. et al. Kinetics of in vivo muscle insulin-mediated glucose uptake in human obesity. Diabetes 39, 965–974 (1990).
Kim, J. A., Montagnani, M., Koh, K. K. & Quon, M. J. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113, 1888–1904 (2006).
Jahn, L. A. et al. Insulin enhances endothelial function throughout the arterial tree in healthy but not metabolic syndrome subjects. J. Clin. Endocrinol. Metab. 101, 1198–1206 (2016).
Steinberg, H. O., Brechtel, G., Johnson, A., Fineberg, N. & Baron, A. D. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J. Clin. Invest. 94, 1172–1179 (1994). This study showed for the first time that insulin effects on all levels of the vascular tree are impaired in patients with insulin resistance.
Jialal, I. et al. Characterization of the receptors for insulin and the insulin-like growth factors on micro- and macrovascular tissues. Endocrinology 117, 1222–1229 (1985).
Montero, D. Hemodynamic actions of insulin: beyond the endothelium. Front. Physiol. 4, 389 (2013).
King, G. L. & Johnson, S. M. Receptor-mediated transport of insulin across endothelial cells. Science 227, 1583–1586 (1985).
Kubota, T. et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 13, 294–307 (2011).
Azizi, P. M. et al. Clathrin-dependent entry and vesicle-mediated exocytosis define insulin transcytosis across microvascular endothelial cells. Mol. Biol. Cell 26, 740–750 (2015). This study shows the detailed molecular mechanism of insulin transcytosis through the endothelial layer.
Wang, H., Wang, A. X., Aylor, K. & Barrett, E. J. Nitric oxide directly promotes vascular endothelial insulin transport. Diabetes 62, 4030–4042 (2013).
Symons, J. D. et al. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ. Res. 104, 1085–1094 (2009).
Muniyappa, R., Iantorno, M. & Quon, M. J. An integrated view of insulin resistance and endothelial dysfunction. Endocrinol. Metab. Clin. North Am. 37, 685–711, (2008).
Wang, Y. et al. APPL1 counteracts obesity-induced vascular insulin resistance and endothelial dysfunction by modulating the endothelial production of nitric oxide and endothelin-1 in mice. Diabetes 60, 3044–3054 (2011).
Ryu, J. et al. APPL1 potentiates insulin sensitivity by facilitating the binding of IRS1/2 to the insulin receptor. Cell Rep. 7, 1227–1238 (2014).
Du, K., Herzig, S., Kulkarni, R. N. & Montminy, M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 300, 1574–1577 (2003).
de Boer, M. P. et al. Globular adiponectin controls insulin-mediated vasoreactivity in muscle through AMPKα2. Vascul Pharmacol. 78, 24–35 (2016).
Dong, Z. et al. Protein kinase A mediates glucagon-like peptide 1-induced nitric oxide production and muscle microvascular recruitment. Am. J. Physiol. Endocrinol. Metab. 304, E222–E228 (2013).
Wang, B. et al. Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes Obes. Metab. 15, 737–749 (2013).
Vicent, D. et al. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J. Clin. Invest. 111, 1373–1380 (2003).
Duplain, H. et al. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation 104, 342–345 (2001).
Abe, H. et al. Hypertension, hypertriglyceridemia, and impaired endothelium-dependent vascular relaxation in mice lacking insulin receptor substrate-1. J. Clin. Invest. 101, 1784–1788 (1998).
Huang, C. et al. Arg972 insulin receptor substrate-1 inhibits endothelial nitric oxide synthase expression in human endothelial cells by upregulating microRNA-155. Int. J. Mol. Med. 36, 239–248 (2015).
Hashimoto, S. et al. Insulin receptor substrate-2 (Irs2) in endothelial cells plays a crucial role in insulin secretion. Diabetes 64, 876–886 (2015).
Hayashi, K. et al. Effects of insulin on rat renal microvessels: studies in the isolated perfused hydronephrotic kidney. Kidney Int. 51, 1507–1513 (1997).
Schmetterer, L. et al. Renal and ocular hemodynamic effects of insulin. Diabetes 46, 1868–1874 (1997).
Hayashi, K. et al. Altered renal microvascular response in Zucker obese rats. Metabolism 51, 1553–1561 (2002).
Buscemi, S. et al. Intra-renal hemodynamics and carotid intima-media thickness in the metabolic syndrome. Diabetes Res. Clin. Pract. 86, 177–185 (2009).
Novikov, A. & Vallon, V. Sodium glucose cotransporter 2 inhibition in the diabetic kidney: an update. Curr. Opin. Nephrol. Hypertens. 25, 50–58 (2016).
Siegel-Axel, D. I. & Haring, H. U. Perivascular adipose tissue: An unique fat compartment relevant for the cardiometabolic syndrome. Rev. Endocr. Metab. Disord. 17, 51–60 (2016). This review describes the interactions of perivascular fat at different anatomical locations on the underlying vessel wall.
Tano, J. Y., Schleifenbaum, J. & Gollasch, M. Perivascular adipose tissue, potassium channels, and vascular dysfunction. Arterioscler Thromb. Vasc. Biol. 34, 1827–1830 (2014).
Gil-Ortega, M., Somoza, B., Huang, Y., Gollasch, M. & Fernandez-Alfonso, M. S. Regional differences in perivascular adipose tissue impacting vascular homeostasis. Trends Endocrinol. Metab. 26, 367–375 (2015).
Rittig, K. et al. The secretion pattern of perivascular fat cells is different from that of subcutaneous and visceral fat cells. Diabetologia 55, 1514–1525 (2012).
Siegel-Axel, D. I. et al. Fetuin-A influences vascular cell growth and production of proinflammatory and angiogenic proteins by human perivascular fat cells. Diabetologia 57, 1057–1066 (2014).
Gao, Y. J., Lu, C., Su, L. Y., Sharma, A. M. & Lee, R. M. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br. J. Pharmacol. 151, 323–331 (2007).
van den Born, J. C., Hammes, H. P., Greffrath, W., van Goor, H. & Hillebrands, J. L. Gasotransmitters in Vascular Complications of Diabetes. Diabetes 65, 331–345 (2016).
Houben, A. J. et al. Perivascular fat and the microcirculation: relevance to insulin resistance, diabetes, and cardiovascular disease. Curr. Cardiovasc. Risk Rep. 6, 80–90 (2012). This article emphasises the possible roles of perivascular fat in vascular dysfunction.
Yudkin, J. S., Eringa, E. & Stehouwer, C. D. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet 365, 1817–1820 (2005).
Rittig, K. et al. Perivascular fatty tissue at the brachial artery is linked to insulin resistance but not to local endothelial dysfunction. Diabetologia 51, 2093–2099 (2008).
de Vries, A. P. et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2, 417–426 (2014).
Foster, M. C. et al. Fatty kidney, hypertension, and chronic kidney disease: the Framingham Heart Study. Hypertension 58, 784–790 (2011).
Lamacchia, O. et al. Para- and perirenal fat thickness is an independent predictor of chronic kidney disease, increased renal resistance index and hyperuricaemia in type-2 diabetic patients. Nephrol. Dial. Transplant. 26, 892–898 (2011).
Wagner, R. et al. Exercise-induced albuminuria is associated with perivascular renal sinus fat in individuals at increased risk of type 2 diabetes. Diabetologia 55, 2054–2058 (2012).
Hysing, J., Ostensen, J., Tolleshaug, H., Andersen, K. J. & Kiil, F. Luminal and basolateral uptake and degradation of insulin in the proximal tubules of the dog kidney. Acta Physiol. Scand. 146, 241–250 (1992).
ter Maaten, J. C. et al. Insulin's acute effects on glomerular filtration rate correlate with insulin sensitivity whereas insulin's acute effects on proximal tubular sodium reabsorption correlation with salt sensitivity in normal subjects. Nephrol. Dial. Transplant. 14, 2357–2363 (1999).
Hiromura, K., Monkawa, T., Petermann, A. T., Durvasula, R. V. & Shankland, S. J. Insulin is a potent survival factor in mesangial cells: role of the PI3-kinase/Akt pathway. Kidney Int. 61, 1312–1321 (2002).
Foutz, R. M., Grimm, P. R. & Sansom, S. C. Insulin increases the activity of mesangial BK channels through MAPK signaling. Am. J. Physiol. Renal Physiol. 294, F1465–1472 (2008).
Thameem, F. et al. The Gly(972)Arg variant of human IRS1 gene is associated with variation in glomerular filtration rate likely through impaired insulin receptor signaling. Diabetes 61, 2385–2393 (2012).
Yano, N. et al. In vitro silencing of the insulin receptor attenuates cellular accumulation of fibronectin in renal mesangial cells. Cell Commun. Signal. 10, 29 (2012).
Isshiki, K. et al. Insulin regulates SOCS2 expression and the mitogenic effect of IGF-1 in mesangial cells. Kidney Int. 74, 1434–1443 (2008).
Kong, Y. L. et al. Insulin deficiency induces rat renal mesangial cell dysfunction via activation of IGF-1/IGF-1R pathway. Acta Pharmacol. Sin. 37, 217–227 (2016).
Weigert, C. et al. Evidence for a novel TGF-beta1-independent mechanism of fibronectin production in mesangial cells overexpressing glucose transporters. Diabetes 52, 527–535 (2003).
Coward, R. J. et al. Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes 56, 1127–1135 (2007).
Kim, E. Y., Anderson, M. & Dryer, S. E. Insulin increases surface expression of TRPC6 channels in podocytes: role of NADPH oxidases and reactive oxygen species. Am. J. Physiol. Renal Physiol. 302, F298–F307 (2012).
Kim, E. Y. & Dryer, S. E. Effects of insulin and high glucose on mobilization of slo1 BKCa channels in podocytes. J. Cell. Physiol. 226, 2307–2315 (2011).
Tejada, T. et al. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int. 73, 1385–1393 (2008).
Welsh, G. I. et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 12, 329–340 (2010). This study shows podocyte loss upon disruption of insulin signalling, highlighting the essential role of insulin in podocyte health and viability.
Madhusudhan, T. et al. Defective podocyte insulin signalling through p85-XBP1 promotes ATF6-dependent maladaptive ER-stress response in diabetic nephropathy. Nat. Commun. 6, 6496 (2015).
Baum, M. Insulin stimulates volume absorption in the rabbit proximal convoluted tubule. J. Clin. Invest. 79, 1104–1109 (1987).
Takahashi, N., Ito, O. & Abe, K. Tubular effects of insulin. Hypertens. Res. 19 (Suppl. 1), S41–S45 (1996).
DeFronzo, R. A., Goldberg, M. & Agus, Z. S. The effects of glucose and insulin on renal electrolyte transport. J. Clin. Invest. 58, 83–90 (1976).
Nizet, A., Lefebvre, P. & Crabbe, J. Control by insulin of sodium potassium and water excretion by the isolated dog kidney. Pflugers Arch. 323, 11–20 (1971).
Brands, M. W., Hildebrandt, D. A., Mizelle, H. L. & Hall, J. E. Sustained hyperinsulinemia increases arterial pressure in conscious rats. Am. J. Physiol. 260, R764–R768 (1991).
Brands, M. W. & Manhiani, M. M. Sodium-retaining effect of insulin in diabetes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R1101–R1109 (2012). This review unravels the controversy regarding the role of insulin in sodium transport in vivo.
Manhiani, M. M., Cormican, M. T. & Brands, M. W. Chronic sodium-retaining action of insulin in diabetic dogs. Am. J. Physiol. Renal Physiol. 300, F957–F965 (2011).
Blazer-Yost, B. L., Esterman, M. A. & Vlahos, C. J. Insulin-stimulated trafficking of ENaC in renal cells requires PI 3-kinase activity. Am. J. Physiol. Cell Physiol. 284, C1645–C1653 (2003).
Lang, F., Artunc, F. & Vallon, V. The physiological impact of the serum and glucocorticoid-inducible kinase SGK1. Curr. Opin. Nephrol. Hypertens. 18, 439–448 (2009).
Lang, F. et al. Deranged transcriptional regulation of cell-volume-sensitive kinase hSGK in diabetic nephropathy. Proc. Natl Acad. Sci. USA 97, 8157–8162 (2000).
Tiwari, S. et al. Impaired sodium excretion and increased blood pressure in mice with targeted deletion of renal epithelial insulin receptor. Proc. Natl Acad. Sci. USA 105, 6469–6474 (2008).
Li, L., Garikepati, R. M., Tsukerman, S., Tiwari, S. & Ecelbarger, C. M. Salt sensitivity of nitric oxide generation and blood pressure in mice with targeted knockout of the insulin receptor from the renal tubule. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R505–R512 (2012).
Li, L. et al. Reduced ENaC activity and blood pressure in mice with genetic knockout of the insulin receptor in the renal collecting duct. Am. J. Physiol. Renal Physiol. 304, F279–F288 (2013).
Pavlov, T. S. et al. Regulation of ENaC in mice lacking renal insulin receptors in the collecting duct. FASEB J. 27, 2723–2732 (2013). This paper shows decreased ENaC activity in mice that lack the insulin receptor in the AQP2-expressing distal tubule.
Stumvoll, M., Meyer, C., Mitrakou, A. & Gerich, J. E. Important role of the kidney in human carbohydrate metabolism. Med. Hypotheses 52, 363–366 (1999).
Tiwari, S. et al. Deletion of the insulin receptor in the proximal tubule promotes hyperglycemia. J. Am. Soc. Nephrol. 24, 1209–1214 (2013).
Eid, A. et al. Intrinsic gluconeogenesis is enhanced in renal proximal tubules of Zucker diabetic fatty rats. J. Am. Soc. Nephrol. 17, 398–405 (2006).
Ghezzi, C. & Wright, E. M. Regulation of the human Na+-dependent glucose cotransporter hSGLT2. Am. J. Physiol. Cell Physiol. 303, C348–C354 (2012).
Vallon, V. et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am. J. Physiol. Renal Physiol. 304, F156–F167 (2013).
Wilding, J. P. The role of the kidneys in glucose homeostasis in type 2 diabetes: clinical implications and therapeutic significance through sodium glucose co-transporter 2 inhibitors. Metabolism 63, 1228–1237 (2014).
Accili, D. et al. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat. Genet. 12, 106–109 (1996).
Joshi, R. L. et al. Targeted disruption of the insulin receptor gene in the mouse results in neonatal lethality. EMBO j 15, 1542–1547 (1996).
Accili, D. Insulin Receptor Knock-Out Mice. Trends Endocrinol. Metabolism 8, 101–104 (1997).
Brüning, J. C. et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell 2, 559–569 (1998).
Michael, M. D. et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell 6, 87–97 (2000).
Mima, A. et al. Glomerular-specific protein kinase C-beta-induced insulin receptor substrate-1 dysfunction and insulin resistance in rat models of diabetes and obesity. Kidney Int. 79, 883–896 (2011). This study investigates insulin signalling in the glomeruli and renal tubules and shows that insulin-resistance occurs only in the glomeruli.
Rocchini, A. P. et al. Insulin and renal sodium retention in obese adolescents. Hypertension 14, 367–374 (1989).
Skott, P. et al. Effect of insulin on renal sodium handling in hyperinsulinaemic type 2 (non-insulin-dependent) diabetic patients with peripheral insulin resistance. Diabetologia 34, 275–281 (1991).
Nakamura, M. et al. Stimulatory effect of insulin on renal proximal tubule sodium transport is preserved in type 2 diabetes with nephropathy. Biochem. Biophys. Res. Commun. 461, 154–158 (2015).
Nakamura, M. et al. Preserved Na/HCO3 cotransporter sensitivity to insulin may promote hypertension in metabolic syndrome. Kidney Int. 87, 535–542 (2015).
Grahammer, F. et al. mTORC2 critically regulates renal potassium handling. J. Clin. Invest. 126, 1773–1782 (2016). This study proves that mTORC2 is the hydrophobic motif kinase of SGK1.
Gerich, J. E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet. Med. 27, 136–142 (2010).
Meyer, C. et al. Abnormal renal and hepatic glucose metabolism in type 2 diabetes mellitus. J. Clin. Invest. 102, 619–624 (1998). This study demonstrates insulin resistance of renal gluconeogenesis in patients with type 2 diabetes.
Zheng, Y. et al. Roles of insulin receptor substrates in insulin-induced stimulation of renal proximal bicarbonate absorption. J. Am. Soc. Nephrol. 16, 2288–2295 (2005).
Schafer, S. et al. Lifestyle intervention in individuals with normal versus impaired glucose tolerance. Eur. J. Clin. Invest. 37, 535–543 (2007).
Machann, J. et al. Follow-up whole-body assessment of adipose tissue compartments during a lifestyle intervention in a large cohort at increased risk for type 2 diabetes. Radiology 257, 353–363 (2010).
Stefan, N. et al. A high-risk phenotype associates with reduced improvement in glycaemia during a lifestyle intervention in prediabetes. Diabetologia 58, 2877–2884 (2015).
Cohen, J. B. & Cohen, D. L. Cardiovascular and renal effects of weight reduction in obesity and the metabolic syndrome. Curr. Hypertens. Rep. 17, 34 (2015).
Rocchini, A. P. et al. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N. Engl. J. Med. 321, 580–585 (1989).
Lavrencic, A., Salobir, B. G. & Keber, I. Physical training improves flow-mediated dilation in patients with the polymetabolic syndrome. Arterioscler Thromb. Vasc. Biol. 20, 551–555 (2000).
Vinet, A. et al. Impact of a lifestyle program on vascular insulin resistance in metabolic syndrome subjects: the RESOLVE study. J. Clin. Endocrinol. Metab. 100, 442–450 (2015).
Thamer, C. et al. High visceral fat mass and high liver fat are associated with resistance to lifestyle intervention. Obesity (Silver Spring) 15, 531–538 (2007).
Fenske, W. et al. Obesity-related cardiorenal disease: the benefits of bariatric surgery. Nat. Rev. Nephrol. 9, 539–551 (2013).
American Diabetes Association. Approaches to glycemic treatment. Diabetes Care 39 (Suppl. 1), S52–S59 (2016).
Sarafidis, P. A. & Lasaridis, A. N. Actions of peroxisome proliferator–activated receptors–γ agonists explaining a possible blood pressure–lowering effect. Am. J. Hypertension 19, 646–653 (2006).
Sarafidis, P. A., Stafylas, P. C., Georgianos, P. I., Saratzis, A. N. & Lasaridis, A. N. Effect of thiazolidinediones on albuminuria and proteinuria in diabetes: a meta-analysis. Am. J. Kidney Dis. 55, 835–847 (2010).
Dagenais, G. R. et al. Effects of ramipril and rosiglitazone on cardiovascular and renal outcomes in people with impaired glucose tolerance or impaired fasting glucose: results of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial. Diabetes Care 31, 1007–1014 (2008).
Artunc, F. et al. Lack of the serum and glucocorticoid-inducible kinase SGK1 attenuates the volume retention after treatment with the PPARgamma agonist pioglitazone. Pflugers Arch. 456, 425–436 (2008).
Ochi, A. et al. Direct inhibitory effects of pioglitazone on hepatic fetuin-A expression. PLoS ONE 9, e88704 (2014).
Mori, K. et al. Effects of pioglitazone on serum fetuin-A levels in patients with type 2 diabetes mellitus. Metabolism 57, 1248–1252 (2008).
Poitout, V. & Robertson, R. P. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr. Rev. 29, 351–366 (2008).
Bensellam, M., Laybutt, D. R. & Jonas, J. C. The molecular mechanisms of pancreatic beta-cell glucotoxicity: recent findings and future research directions. Mol. Cell Endocrinol. 364, 1–27 (2012).
Kaul, K., Apostolopoulou, M. & Roden, M. Insulin resistance in type 1 diabetes mellitus. Metabolism 64, 1629–1639 (2015).
Hanefeld, M., Monnier, L., Schnell, O. & Owens, D. Early treatment with basal insulin glargine in people with type 2 diabetes: lessons from ORIGIN and other cardiovascular trials. Diabetes Ther. 7, 187–201 (2016).
Gerstein, H. C. et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med. 367, 319–328 (2012).
Gilbert, R. E. et al. Basal insulin glargine and microvascular outcomes in dysglycaemic individuals: results of the Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial. Diabetologia 57, 1325–1331 (2014).
Ferrannini, E. et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Invest. 124, 499–508 (2014).
Merovci, A. et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J. Clin. Invest. 124, 509–514 (2014).
Zinman, B. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128 (2015).
Wanner, C. et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 375, 323–334 (2016).
Acknowledgements
We acknowledge the meticulous work of Marketa Kovarova (Department of Internal Medicine IV, Division of Endocrinology, Diabetology, Vascular Disease, Nephrology and Clinical Chemistry, University Hospital Tübingen, Germany) in designing the figures. The authors' work is funded by a grant from the German Federal Ministry of Education and Research to the German Centre for Diabetes Research (DZD), München-Neuherberg, Germany.
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, discussed the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Impaired glucose tolerance
-
Defined as a plasma glucose concentration of 140–200 mg/dl (7.77–11.1 mmol/l) measured 2 h after an oral glucose load of 75 g.
- Visceral obesity
-
Increased waist circumference as a result of an accumulation of fat in the intra-abdominal compartments, such as the omentum majus.
- Hepatokines
-
Factors that are secreted from the liver and act on other tissues.
- Hyperinsulinaemic–euglycaemic clamp
-
Test used to quantify insulin resistance on a whole-body level. Continuous insulin infusion is used to maintain plasma insulin levels, whilst variable glucose infusion is used to maintain plasma glucose concentration at basal levels. When a stable plasma glucose concentration is achieved, the rate of glucose infusion is equal to the rate of glucose uptake by all of the body tissues.
- Renal resistive index
-
A measure of intrarenal vascular resistance.
- Kimmelstiel–Wilson lesions
-
The typical histopathological hallmark of diabetic nephropathy, which is characterized by nodular glomerulosclerosis.
- Impaired fasting glucose
-
Defined as a plasma glucose concentration of 110–126 mg/dl (6.11–6.99 mmol/l) in the fasting state.
- Oral glucose tolerance test
-
Test used to screen for disturbances in glucose metabolism and insulin resistance.
- Liver steatosis
-
Accumulation of excess fat in the liver.
- Homeostasis model assessment of insulin resistance
-
A simple quantitative measure of insulin resistance calculated from the plasma fasting glucose level and insulin concentration.
Rights and permissions
About this article
Cite this article
Artunc, F., Schleicher, E., Weigert, C. et al. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol 12, 721–737 (2016). https://doi.org/10.1038/nrneph.2016.145
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2016.145
This article is cited by
-
Whole transcriptome mapping reveals the lncRNA regulatory network of TFP5 treatment in diabetic nephropathy
Genes & Genomics (2024)
-
Triglyceride-glucose index, renal function and cardiovascular disease: a national cohort study
Cardiovascular Diabetology (2023)
-
The relationship and interaction between triglyceride glucose index and obesity in the risk of prehypertension population: a cross-sectional study from a survey in Anhui, Eastern China
BMC Cardiovascular Disorders (2023)
-
Triglyceride glucose index is a significant predictor of severe disturbance of consciousness and all-cause mortality in critical cerebrovascular disease patients
Cardiovascular Diabetology (2023)
-
Identifying a target group for selenium supplementation in high-risk cardiac surgery: a secondary analysis of the SUSTAIN CSX trial
Intensive Care Medicine Experimental (2023)