Key Points
-
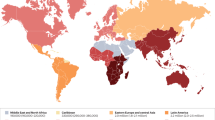
Widespread use of antiretroviral therapy has led to a change in the spectrum of renal pathologies associated with HIV infection
-
The incidence of HIV-associated nephropathy (HIVAN) has decreased since the introduction of combined antiretroviral therapy (cART)
-
Viral factors that likely contribute to renal injury in HIV-positive patients include direct infection of podocytes and renal tubular epithelial cells as well as the HIV proteins Nef and Vpr
-
APOL1 genetic variants predispose to HIVAN but not to HIV-immune-complex kidney disease
-
All HIV-positive individuals should undergo periodic (at least annual) screening of renal function
-
All patients with HIV-associated kidney diseases should receive cART; standard therapies for chronic kidney disease are also recommended
Abstract
HIV is a highly adaptive, rapidly evolving virus, which is associated with renal diseases including collapsing glomerulopathy—the classic histomorphological form of HIV-associated nephropathy. Other nephropathies related to viral factors include HIV-immune-complex kidney disease and thrombotic microangiopathy. The distribution of HIV-associated kidney diseases has changed over time and continues to vary across geographic regions worldwide. The reasons for this diversity are complex and include a critical role of APOL1 variants and possibly other genetic factors, disparities in access to effective antiviral therapies, and likely other factors that we do not yet fully understand. The mechanisms responsible for HIVAN, including HIV infection of podocytes and tubular epithelial cells, the molecules responsible for HIV entry, and diverse mechanisms of cell injury, have been the focus of much study. Although combined antiretroviral therapy is effective at preventing and reversing HIVAN, focal segmental glomerulosclerosis, arterionephrosclerosis and diabetic nephropathy are increasingly common in individuals who have received such therapy for many years. These diseases are associated with metabolic syndrome, obesity and premature ageing. Future directions for HIV-related kidney disease will involve regular screening for drug nephrotoxicity and incipient renal disease, as well as further research into the mechanisms by which chronic inflammation can lead to glomerular disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rao, T. K. et al. Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N. Engl. J. Med. 310, 669–673 (1984).
UNAIDS. UNAIDS Factsheet 2014. unaids.org [online], (2014).
Knobler, S., Mack, A., Mahmoud, A. & Lemon, S. (Eds) The Threat of Pandemic Influenza: Are We Ready? Workshop Summary. (National Academies Press, 2005).
The Henry J. Kaiser Family Foundation. The HIV/AIDS Epidemic in the United States. kff.org [online], (2014).
Mallipattu, S. K., Wyatt, C. M. & He, J. C. The new epidemiology of HIV-related kidney disease. J. AIDS Clin. Res. S4, 001 (2012).
Fabian, J. & Naicker, S. HIV and kidney disease in sub-Saharan Africa. Nat. Rev. Nephrol. 5, 591–598 (2009).
Gerntholtz, T. E., Goetsch, S. J. & Katz, I. HIV-related nephropathy: a South African perspective. Kidney Int. 69, 1885–1891 (2006).
Han, T. M., Naicker, S., Ramdial, P. K. & Assounga, A. G. A cross-sectional study of HIV-seropositive patients with varying degrees of proteinuria in South Africa. Kidney Int. 69, 2243–2250 (2006).
Wearne, N., Swanepoel, C. R., Boulle, A., Duffield, M. S. & Rayner, B. L. The spectrum of renal histologies seen in HIV with outcomes, prognostic indicators and clinical correlations. Nephrol. Dial. Transplant. 27, 4109–4118 (2012).
Mocroft, A. et al. Chronic renal failure among HIV-1-infected patients. AIDS 21, 1119–1127 (2007).
Cheung, C. Y. et al. Prevalence of chronic kidney disease in Chinese HIV-infected patients. Nephrol. Dial. Transplant. 22, 3186–3190 (2007).
Cavalcante, M. A., Coelho, S. N. & Lacerda, H. R. Prevalence of persistent proteinuria in stable HIV/AIDS patients and its association with HIV nephropathy. Braz. J. Infect. Dis. 11, 456–461 (2007).
Hailemariam, S. et al. Renal pathology and premortem clinical presentation of Caucasian patients with AIDS: an autopsy study from the era prior to antiretroviral therapy. Swiss Med. Wkly 131, 412–417 (2001).
Janakiraman, H. et al. Correlation of CD4 counts with renal disease in HIV positive patients. Saudi J. Kidney Dis. Transpl. 19, 603–607 (2008).
US Renal Data System. USRDS Annual Data Report: atlas of end-stage renal disease in the United States. ursds.org [online], (2012).
Lescure, F. X. et al. HIV-associated kidney glomerular diseases: changes with time and HAART. Nephrol. Dial. Transplant. 27, 2349–2355 (2012).
Chandel, N. et al. Renin modulates HIV replication in T cells. J. Leukoc. Biol. 96, 601–609 (2014).
Ryom, L. et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. J. Infect. Dis. 207, 1359–1369 (2013).
Cooper, R. D. et al. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin. Infect. Dis. 51, 496–505 (2010).
Mallipattu, S. K., Salem, F. & Wyatt, C. M. The changing epidemiology of HIV-related chronic kidney disease in the era of antiretroviral therapy. Kidney Int. 86, 259–265 (2014).
Phair, J. & Palella, F. Renal disease in HIV-infected individuals. Curr. Opin. HIV AIDS 6, 285–289 (2011).
Bani-Hani, S. et al. Renal disease in AIDS: it is not always HIVAN. Clin. Exp. Nephrol. 14, 263–267 (2010).
Gardenswartz, M. H. et al. Renal disease in patients with AIDS: a clinicopathologic study. Clin. Nephrol. 21, 197–204 (1984).
Pardo, V. et al. Glomerular lesions in the acquired immunodeficiency syndrome. Ann. Intern. Med. 101, 429–434 (1984).
Chang, B. G., Markowitz, G. S., Seshan, S. V., Seigle, R. L. & D'Agati, V. D. Renal manifestations of concurrent systemic lupus erythematosus and HIV infection. Am. J. Kidney Dis. 33, 441–449 (1999).
Madiwale, C. & Venkataseshan, V. S. Renal lesions in AIDS: a biopsy and autopsy study. Indian J. Pathol. Microbiol. 42, 45–54 (1999).
Kofman, T. et al. Collapsing glomerulopathy associated lupus in a black female with homozygous APOL1 mutation. Lupus 21, 1459–1462 (2012).
Okpechi I. G., Ayodele, O. E., Rayner, B. L. & Swanepoel, C. R. Kidney disease in elderly South Africans. Clin. Nephrol. 79, 269–276 (2013).
Ferreira, A. C., Carvalho, D., Carvalho, F., Galvao, M. J. & Nolasco, F. Collapsing glomerulopathy in Portugal: a review of the histological and clinical findings in HIV and non-HIV patients. Nephrol. Dial. Transplant. 26, 2209–2215 (2011).
Mesquita, M. et al. Renal biopsy findings in Belgium: a retrospective single center analysis. Acta Clin. Belg. 66, 104–109 (2011).
Fine, D. M., Fogo, A. B. & Alpers, C. E. Thrombotic microangiopathy and other glomerular disorders in the HIV-infected patient. Semin. Nephrol. 28, 545–555 (2008).
Haas, M., Kaul, S., & Eustace, J. A. HIV-associated immune complex glomerulonephritis with “lupus-like” features: a clinicopathologic study of 14 cases. Kidney Int. 67, 1381–1390 (2005).
Stokes, M. B. et al. Immune complex glomerulonephritis in patients coinfected with human immunodeficiency virus and hepatitis C virus. Am. J. Kidney Dis. 29, 514–525 (1997).
Nebuloni, M. et al. Glomerular lesions in HIV-positive patients: a 20-year biopsy experience from Northern Italy. Clin. Nephrol. 72, 38–45 (2009).
Haas, M., Rajaraman, S., Ahuja, T., Kittaka, M. & Cavallo, T. Fibrillary/immunotactoid glomerulonephritis in HIV-positive patients: a report of three cases. Nephrol. Dial. Transplant. 15, 1679–1683 (2000).
Chandra, P. & Kopp, J. B. Viruses and collapsing glomerulopathy: a brief critical review. Clin. Kidney J. 6, 1–5 (2013).
Romagnani, P., & Remuzzi, G. Renal progenitors in non-diabetic and diabetic nephropathies. Trends Endocrinol. Metab. 24, 13–20 (2013).
Grahammer, F., Wanner, N. & Huber, T. B. Podocyte regeneration: who can become a podocyte? Am. J. Pathol. 183, 333–335 (2013).
Ray, P. E. et al. Human immunodeficiency virus (HIV)-associated nephropathy in children from the Washington D. C. area: 12 years' experience. Semin. Nephrol. 18, 396–405 (1998).
Anochie, I. C., Eke, F. U. & Okpere, A. N. Human immunodeficiency virus-associated nephropathy (HIVAN) in Nigerian children. Pediatr. Nephrol. 23, 117–122 (2008).
Lanjewar, D. N., Ansari, M. A., Shetty, C. R., Maheshwari, M. B. & Jain, P. Renal lesions associated with AIDS—an autopsy study. Indian J. Pathol. Microbiol. 42, 63–68 (1999).
Williams, D. I. et al. Presentation, pathology, and outcome of HIV associated renal disease in a specialist centre for HIV/AIDS. Sex. Transm. Infect. 74, 179–184 (1998).
Praditpornsilpa, K. et al. Renal pathology and HIV infection in Thailand. Am. J. Kidney Dis. 33, 282–286 (1999).
Cove-Smith, A., Sheaff, M. T. & Ashman, N. HIVAN is increasingly less common in HIV-positive black Africans living in Europe. Kidney Int. 70, 1662 (2006).
Mohan, S. et al. The changing pattern of glomerular disease in HIV and hepatitis C co-infected patients in the era of HAART. Clin. Nephrol. 79, 285–291 (2013).
Foy, M. C. et al. Comparison of risk factors and outcomes in HIV immune complex kidney disease and HIV-associated nephropathy. Clin. J. Am. Soc. Nephrol. 8, 1524–1532 (2013).
Stock, P. G. et al. Outcomes of kidney transplantation in HIV-infected recipients. N. Engl. J. Med. 363, 2004–2014 (2010).
Chandran, S., Jen, K. Y. & Laszik, Z. G. Recurrent HIV-associated immune complex glomerulonephritis with lupus-like features after kidney transplantation. Am. J. Kidney Dis. 62, 335–338 (2013).
Mikulak, J. & Singhal, P. C. HIV-1 and kidney cells: better understanding of viral interaction. Nephron Exp. Nephrol. 115, e15–e21 (2010).
Cohen, A. H., Sun, N. C., Shapshak, P. & Imagawa, D. T. Demonstration of human immunodeficiency virus in renal epithelium in HIV-associated nephropathy. Mod. Pathol. 2, 125–128 (1989).
Eitner, F. et al. Chemokine receptor CCR5 and CXCR4 expression in HIV-associated kidney disease. J. Am. Soc. Nephrol. 11, 856–867 (2000).
Hiramatsu, N. et al. Angiotensin II type 1 receptor blockade inhibits the development and progression of HIV-associated nephropathy in a mouse model. J. Am. Soc. Nephrol. 18, 515–527 (2007).
Simard, M. C. et al. Expression of simian immunodeficiency virus nef in immune cells of transgenic mice leads to a severe AIDS-like disease. J. Virol. 76, 3981–3995 (2002).
Zuo, Y. et al. HIV-1 genes vpr and nef synergistically damage podocytes, leading to glomerulosclerosis. J. Am. Soc. Nephrol. 17, 2832–2843 (2006).
Hatsukari, I. et al. DEC-205-mediated internalization of HIV-1 results in the establishment of silent infection in renal tubular cells. J. Am. Soc. Nephrol. 18, 780–787 (2007).
Singh, P. et al. Tubular cell HIV-entry through apoptosed CD4 T cells: a novel pathway. Virology 434, 68–77 (2012).
Xie, X. et al. The basic domain of HIV-tat transactivating protein is essential for its targeting to lipid rafts and regulating fibroblast growth factor-2 signaling in podocytes isolated from children with HIV-1-associated nephropathy. J. Am. Soc. Nephrol. 25, 1800–1813 (2014).
Khatua, A. K., Taylor, H. E., Hildreth, J. E. & Popik, W. Non-productive HIV-1 infection of human glomerular and urinary podocytes. Virology 408, 119–127 (2010).
Bruggeman, L. A. et al. Nephropathy in human immunodeficiency virus-1 transgenic mice is due to renal transgene expression. J. Clin. Invest. 100, 84–92 (1997).
Ray, P. E. et al. A novel HIV-1 transgenic rat model of childhood HIV-1-associated nephropathy. Kidney Int. 63, 2242–2253 (2003).
Ray, P. E. et al. Infection of human primary renal epithelial cells with HIV-1 from children with HIV-associated nephropathy. Kidney Int. 53, 1217–1229 (1998).
Ross, M. J., Bruggeman, L. A., Wilson, P. D. & Klotman, P. E. Microcyst formation and HIV-1 gene expression occur in multiple nephron segments in HIV-associated nephropathy. J. Am. Soc. Nephrol. 12, 2645–2651 (2001).
Zhong, J. et al. Expression of HIV-1 genes in podocytes alone can lead to the full spectrum of HIV-1-associated nephropathy. Kidney Int. 68, 1048–1060 (2005).
Liapis, H., Romagnani, P. & Anders, H. J. New insights into the pathology of podocyte loss: mitotic catastrophe. Am. J. Pathol. 183, 1364–1374 (2013).
Snyder, A. et al. FAT10: a novel mediator of Vpr-induced apoptosis in human immunodeficiency virus-associated nephropathy. J. Virol. 83, 11983–11988 (2009).
Conaldi, P. G. et al. HIV-1 kills renal tubular epithelial cells in vitro by triggering an apoptotic pathway involving caspase activation and Fas upregulation. J. Clin. Invest. 102, 2041–2049 (1998).
Vashistha, H. et al. HIV-1 expression induces tubular cell G2/M arrest and apoptosis. Ren. Fail. 30, 655–664 (2008).
Alpers, C. E., McClure, J. & Bursten, S. L. Human mesangial cells are resistant to productive infection by multiple strains of human immunodeficiency virus types 1 and 2. Am. J. Kidney Dis. 19, 126–130 (1992).
Tokizawa, S. et al. Infection of mesangial cells with HIV and SIV: identification of GPR1 as a coreceptor. Kidney Int. 58, 607–617 (2000).
Singhal, P. C. et al. HIV-1 gp160 envelope protein modulates proliferation and apoptosis in mesangial cells. Nephron 76, 284–295 (1997).
Yamamoto, T. et al. Increased levels of transforming growth factor-β in HIV-associated nephropathy. Kidney Int. 55, 579–592 (1999).
Chen, G. et al. A protective role for kidney apolipoprotein E. Regulation of mesangial cell proliferation and matrix expansion. J. Biol. Chem. 276, 49142–49147 (2001).
Ahuja, T. S., Gopalani, A., Davies, P. & Ahuja, H. Matrix metalloproteinase-9 expression in renal biopsies of patients with HIV-associated nephropathy. Nephron Clin. Pract. 95, c100–c104 (2003).
Kaufman, L., Hayashi, K., Ross, M. J., Ross, M. D. & Klotman, P. E. Sidekick-1 is upregulated in glomeruli in HIV-associated nephropathy. J. Am. Soc. Nephrol. 15, 1721–1730 (2004).
Kaufman, L. et al. The homophilic adhesion molecule sidekick-1 contributes to augmented podocyte aggregation in HIV-associated nephropathy. FASEB J. 21, 1367–1375 (2007).
Conaldi, P. G. et al. Human immunodeficiency virus-1 tat induces hyperproliferation and dysregulation of renal glomerular epithelial cells. Am. J. Pathol. 161, 53–61 (2002).
Sunamoto, M., Husain, M., He, J. C., Schwartz, E. J. & Klotman, P. E. Critical role for Nef in HIV-1-induced podocyte dedifferentiation. Kidney Int. 64, 1695–1701 (2003).
Barisoni, L., Bruggeman, L. A., Mundel, P., D'Agati, V. D. & Klotman, P. E. HIV-1 induces renal epithelial dedifferentiation in a transgenic model of HIV-associated nephropathy. Kidney Int. 58, 173–181 (2000).
Husain, M., D'Agati, V. D., He, J. C., Klotman, M. E. & Klotman P. E. HIV-1 Nef induces dedifferentiation of podocytes in vivo: a characteristic feature of HIVAN. AIDS 19, 1975–1980 (2005).
Zerhouni-Layachi, B. et al. Dual tropism of HIV-1 envelopes derived from renal tubular epithelial cells of patients with HIV-associated nephropathy. AIDS 20, 621–624 (2006).
Yadav, A. et al. HIVAN phenotype: consequence of epithelial mesenchymal transdifferentiation. Am. J. Physiol. Renal Physiol. 298, F734–F744 (2010).
He, J. C. et al. Nef stimulates proliferation of glomerular podocytes through activation of Src-dependent Stat3 and MAPK1,2 pathways. J. Clin. Invest. 114, 643–651 (2004).
Feng, X. et al. Reduction of Stat3 activity attenuates HIV-induced kidney injury. J. Am. Soc. Nephrol. 20, 2138–2146 (2009).
Gu, L. et al. Deletion of podocyte STAT3 mitigates the entire spectrum of HIV-1-associated nephropathy. AIDS 27, 1091–1098 (2013).
Yadav, A. et al. Sirolimus modulates HIVAN phenotype through inhibition of epithelial mesenchymal transition. Exp. Mol. Pathol. 93, 173–181 (2012).
Rai, P. et al. Rapamycin-induced modulation of HIV gene transcription attenuates progression of HIVAN. Exp. Mol. Pathol. 94, 255–261 (2013).
Sharma, M. et al. Activation of Notch signaling pathway in HIV-associated nephropathy. AIDS 24, 2161–2170 (2010).
Sharma, M. et al. Inhibition of Notch pathway attenuates the progression of human immunodeficiency virus-associated nephropathy. Am. J. Physiol. Renal Physiol. 304, F1127–F1136 (2013).
Barisoni, L. et al. Podocyte cell cycle regulation and proliferation in collapsing glomerulopathies. Kidney Int. 58, 137–143 (2000).
Shankland, S. J. et al. Differential expression of cyclin-dependent kinase inhibitors in human glomerular disease: role in podocyte proliferation and maturation. Kidney Int. 58, 674–683 (2000).
Rosenstiel, P. E. et al. HIV-1 Vpr activates the DNA damage response in renal tubule epithelial cells. AIDS 23, 2054–2056 (2009).
Snyder, A. et al. HIV-1 viral protein r induces ERK and caspase-8-dependent apoptosis in renal tubular epithelial cells. AIDS 24, 1107–1119 (2010).
Ross, M. J. et al. Role of ubiquitin-like protein FAT10 in epithelial apoptosis in renal disease. J. Am. Soc. Nephrol. 17, 996–1004 (2006).
Ross, M. J., Martinka, S., D'Agati, V. D. & Bruggeman, L. A. NF-κB regulates Fas-mediated apoptosis in HIV-associated nephropathy. J. Am. Soc. Nephrol. 16, 2403–2411 (2005).
Martinka, S. & Bruggeman, L. A. Persistent NF-κB activation in renal epithelial cells in a mouse model of HIV-associated nephropathy. Am. J. Physiol. Renal Physiol. 290, F657–F665 (2006).
Rai, P. et al. mTOR plays a critical role in p53-induced oxidative kidney cell injury in HIVAN. Am. J. Physiol. Renal Physiol. 305, F343–F354 (2013).
Doublier, S. et al. HIV-1 Tat reduces nephrin in human podocytes: a potential mechanism for enhanced glomerular permeability in HIV-associated nephropathy. AIDS 21, 423–432 (2007).
Cheng, K. et al. MicroRNAs in HIV-associated nephropathy (HIVAN). Exp. Mol. Pathol. 94, 65–72 (2013).
Cheng, K. et al. Rapamycin-induced modulation of miRNA expression is associated with amelioration of HIV-associated nephropathy (HIVAN). Exp. Cell Res. 319, 2073–2080 (2013).
Chandel, N. et al. HIV compromises integrity of the podocyte actin cytoskeleton through downregulation of the vitamin D receptor. Am. J. Physiol. Renal Physiol. 304, F1347–F1357 (2013).
Stephens, E. B., Tian, C., Dalton, S. B. & Gattone, V. H. 2nd. Simian-human immunodeficiency virus-associated nephropathy in macaques. AIDS Res. Hum. Retroviruses 16, 1295–1306 (2000).
Kopp, J. B. et al. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc. Natl Acad. Sci. USA 89, 1577–1581 (1992).
Reid, W. et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc. Natl Acad. Sci. USA 98, 9271–9276 (2001).
Genovese, G. et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329, 841–845 (2010).
Tzur, S. et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum. Genet. 128, 345–350 (2010).
Limou, S., Nelson, G. W., Kopp, J. B. & Winkler, C. A. APOL1 kidney risk alleles: population genetics and disease associations. Adv. Chronic Kidney Dis. 21, 426–433 (2014).
Behar, D. M. et al. Absence of APOL1 risk variants protects against HIV-associated nephropathy in the Ethiopian population. Am. J. Nephrol. 34, 452–459 (2011).
Kopp, J. B. et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. JASN 22, 2129–2137 (2011).
Kasembeli, A. N. et al. APOL1 risk variants are strongly associated with HIVAN in black South Africans. J. Am. Soc. Nephrol. (in press).
Lan, X. et al. APOL1 risk variants enhance podocyte necrosis through compromising lysosomal membrane permeability. Am. J. Physiol. Renal Physiol. 307, F326–F336 (2014).
Nichols, B. et al. Innate immunity pathways regulate the nephropathy gene apolipoprotein L1. Kidney Int. 87, 332–342 (2015).
Lucas, G. M. et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin. Infect. Dis. 59, e96–e138 (2014).
Berliner, A. R. et al. Observations on a cohort of HIV-infected patients undergoing native renal biopsy. Am. J. Nephrol. 28, 478–486 (2008).
Bohmart, A. & Burns, G. Renal disease in an urban HIV population in the era prior and following the introduction of highly active antiretroviral therapy. J. Natl Med. Assoc. 103, 513–517 (2011).
Abraham, A. G. et al. End-stage renal disease among HIV-infected adults in North America. Clin. Infect. Dis. http://dx.doi.org/10.1093/cid/ciu919.
Becker, S. et al. HIV-associated thrombotic microangiopathy in the era of highly active antiretroviral therapy: an observational study. Clin. Infect. Dis. 39 (Suppl. 5), S267–S275 (2004).
Stohr, W. et al. Glomerular dysfunction and associated risk factors over 4–5 years following antiretroviral therapy initiation in Africa. Antivir. Ther. 16, 1011–1020 (2011).
Peters, P. J. et al. Antiretroviral therapy improves renal function among HIV-infected Ugandans. Kidney Int. 74, 925–929 (2008).
Mpondo, B. C. et al. Impact of antiretroviral therapy on renal function among HIV-infected Tanzanian adults: a retrospective cohort study. PLoS ONE 9, e89573 (2014).
Berns, J. S. & Kasbekar, N. Highly active antiretroviral therapy and the kidney: an update on antiretroviral medications for nephrologists. Clin. J. Am. Soc. Nephrol. 1, 117–129 (2006).
Izzedine, H., Launay-Vacher, V., Baumelou, A. & Deray, G. An appraisal of antiretroviral drugs in hemodialysis. Kidney Int. 60, 821–830 (2001).
Eustace, J. A. et al. Cohort study of the treatment of severe HIV-associated nephropathy with corticosteroids. Kidney Int. 58, 1253–1260 (2000).
Ingulli, E. et al. Nephrotic syndrome associated with acquired immunodeficiency syndrome in children. J. Pediatr. 119, 710–716 (1991).
Gupta, S. K. et al. Guidelines for the management of chronic kidney disease in HIV-infected patients: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin. Infect. Dis. 40, 1559–1585 (2005).
Bige, N. et al. Presentation of HIV-associated nephropathy and outcome in HAART-treated patients. Nephrol. Dial. Transplant. 27, 1114–1121 (2012).
Post, F. A. et al. Predictors of renal outcome in HIV-associated nephropathy. Clin. Infect. Dis. 46, 1282–1289 (2008).
Fine, D. M. et al. APOL1 risk variants predict histopathology and progression to ESRD in HIV-related kidney disease. J. Am. Soc. Nephrol. 23, 343–350 (2012).
Kumar, D. et al. Inhibition of renin activity slows down the progression of HIV-associated nephropathy. Am. J. Physiol. Renal Physiol. 303, F711–F720 (2012).
Kalayjian, R. C. The treatment of HIV-associated nephropathy. Adv. Chronic Kidney Dis. 17, 59–71 (2010).
Ahuja, T. S., Grady, J. & Khan S. Changing trends in the survival of dialysis patients with human immunodeficiency virus in the United States. J. Am. Soc. Nephrol. 13, 1889–1893 (2002).
Macrae, J., Friedman, A. L., Eggers, P. & Friedman, E. A. Improved survival in HIV-infected African-Americans with ESRD. Clin. Nephrol. 64, 124–128 (2005).
Tourret, J. et al. Outcome and prognosis factors in HIV-infected hemodialysis patients. Clin. J. Am. Soc. Nephrol. 1, 1241–1247 (2006).
Vermeulen, A. MMed Thesis, University of Witwatersrand (2014).
Meehan, S. M., Kim, L. & Chang, A. A spectrum of morphologic lesions of focal segmental glomerulosclerosis by Columbia criteria in human immunodeficiency virus infection. Virchows Arch. 460, 429–435 (2012).
Acknowledgements
The authors' work is supported in part by the Intramural Research Programs of the National Cancer Institute, National Institute of Diabetes and Digestive and Kidney Diseases, and National Institutes of Health, USA, and by the Medical Research Council and National Research Foundation of South Africa. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E. Content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, USA, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. The authors note with regret the recent untimely death of Linda Kao, a pioneering and generous researcher who made many important contributions to the field of human genetics, in particular to the discovery of the chromosome 22 locus that includes APOL1 as a risk factor for HIVAN.
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Rosenberg, A., Naicker, S., Winkler, C. et al. HIV-associated nephropathies: epidemiology, pathology, mechanisms and treatment. Nat Rev Nephrol 11, 150–160 (2015). https://doi.org/10.1038/nrneph.2015.9
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2015.9
This article is cited by
-
Steroid resistant nephrotic syndrome with collapsing focal segmental glomerulosclerosis in a 12-year-old Japanese female after SARS-CoV-2 vaccination
Journal of Nephrology (2023)
-
Incidence of impaired kidney function among people with HIV: a systematic review and meta-analysis
BMC Nephrology (2022)
-
Trauma patients with human immunodeficiency virus (HIV): a propensity matched analysis
European Journal of Trauma and Emergency Surgery (2022)
-
Prevalence and risk factors of chronic kidney disease in an HIV positive Mexican cohort
BMC Nephrology (2021)
-
Renal function in Ethiopian HIV-positive adults on antiretroviral treatment with and without tenofovir
BMC Infectious Diseases (2020)