Key Points
-
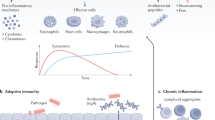
The innate immune system is essential for preventing urinary tract infection (UTI) and limiting the spread of infection
-
Urinary antimicrobial peptides exhibit bactericidal and bacteriostatic activity toward uropathogenic bacteria
-
Intercalated cells are the major renal source of antimicrobial peptides
-
Antimicrobial peptides possess immunomodulatory activity in addition to antimicrobial activity
-
Urinary antimicrobial peptides have diagnostic and therapeutic potential for patients with UTIs
Abstract
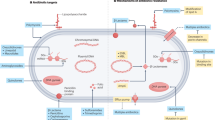
Urinary tract infections (UTIs), including pyelonephritis, are among the most common and serious infections encountered in nephrology practice. UTI risk is increased in selected patient populations with renal and urinary tract disorders. As the prevalence of antibiotic-resistant uropathogens increases, novel and alternative treatment options will be needed to reduce UTI-associated morbidity. Discoveries over the past decade demonstrate a fundamental role for the innate immune system in protecting the urothelium from bacterial challenge. Antimicrobial peptides, an integral component of this urothelial innate immune system, demonstrate potent bactericidal activity toward uropathogens and might represent a novel class of UTI therapeutics. The urothelium of the bladder and the renal epithelium secrete antimicrobial peptides into the urinary stream. In the kidney, intercalated cells—a cell-type involved in acid–base homeostasis—have been shown to be an important source of antimicrobial peptides. Intercalated cells have therefore become the focus of new investigations to explore their function during pyelonephritis and their role in maintaining urinary tract sterility. This Review provides an overview of UTI pathogenesis in the upper and lower urinary tract. We describe the role of intercalated cells and the innate immune response in preventing UTI, specifically highlighting the role of antimicrobial peptides in maintaining urinary tract sterility.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hooton, T. M. Clinical practice. Uncomplicated urinary tract infection. N. Engl. J. Med. 366, 1028–1037 (2012).
Healthcare Cost and Utilization Project. National Inpatient Sample [online], (2012).
Foxman, B., Barlow, R., D'Arcy, H., Gillespie, B. & Sobel, J. D. Urinary tract infection: self-reported incidence and associated costs. Ann. Epidemiol. 10, 509–515 (2000).
Foxman, B. et al. Risk factors for second urinary tract infection among college women. Am. J. Epidemiol. 151, 1194–1205 (2000).
Foxman, B. Recurring urinary tract infection: incidence and risk factors. Am. J. Public Health 80, 331–333 (1990).
Ronald, A. The etiology of urinary tract infection: traditional and emerging pathogens. Am. J. Med. 113 (Suppl. 1A), 14S–19S (2002).
Mulvey, M. A., Schilling, J. D., Martinez, J. J. & Hultgren, S. J. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc. Natl Acad. Sci. USA 97, 8829–8835 (2000).
Becknell, B., Schober, M., Korbel, L. & Spencer, J. D. The diagnosis, evaluation and treatment of acute and recurrent pediatric urinary tract infections. Expert Rev. Anti Infect. Ther. 13, 81–90 (2015).
Harrington, R. D. & Hooton, T. M. Urinary tract infection risk factors and gender. J. Gend. Specif. Med. 3, 27–34 (2000).
Abbott, K. C. et al. Late urinary tract infection after renal transplantation in the United States. Am. J. Kidney Dis. 44, 353–362 (2004).
Alangaden, G. J. et al. Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clin. Transplant. 20, 401–409 (2006).
Pelle, G. et al. Acute pyelonephritis represents a risk factor impairing long-term kidney graft function. Am. J. Transplant. 7, 899–907 (2007).
Zasloff, M. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J. Am. Soc. Nephrol. 18, 2810–2816 (2007).
Spencer, J. D., Schwaderer, A. L., Becknell, B., Watson, J. & Hains, D. S. The innate immune response during urinary tract infection and pyelonephritis. Pediatr. Nephrol. 29, 1139–1149 (2013).
Hato, T. & Dagher, P. C. How the innate immune system senses trouble and causes trouble. Clin. J. Am. Soc. Nephrol. http://dx.doi.org/10.2215/CJN.04680514
Jorgensen, I. & Seed, P. C. How to make it in the urinary tract: a tutorial by Escherichia coli. PLoS Pathog. 8, e1002907 (2012).
Justice, S. S., Harrison, A., Becknell, B. & Mason, K. M. Bacterial differentiation, development, and disease: mechanisms for survival. FEMS Microbiol. Lett. 360, 1–8 (2014).
Dhakal, B. K., Kulesus, R. R. & Mulvey, M. A. Mechanisms and consequences of bladder cell invasion by uropathogenic Escherichia coli. Eur. J. Clin. Invest. 38 (Suppl. 2), 2–11 (2008).
Bishop, B. L. et al. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat. Med. 13, 625–630 (2007).
Justice, S. S. et al. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl Acad. Sci. USA 101, 1333–1338 (2004).
Hunstad, D. A. & Justice, S. S. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu. Rev. Microbiol. 64, 203–221 (2010).
Rosen, D. A., Hooton, T. M., Stamm, W. E., Humphrey, P. A. & Hultgren, S. J. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 4, e329 (2007).
Mysorekar, I. U. & Hultgren, S. J. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl Acad. Sci. USA 103, 14170–14175 (2006).
Schilling, J. D., Lorenz, R. G. & Hultgren, S. J. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect. Immun. 70, 7042–7049 (2002).
Chassin, C. et al. Renal collecting duct epithelial cells react to pyelonephritis-associated Escherichia coli by activating distinct TLR4-dependent and independent inflammatory pathways. J. Immunol. 177, 4773–4784 (2006).
Chassin, C., Tourneur, E., Bens, M. & Vandewalle, A. A role for collecting duct epithelial cells in renal antibacterial defences. Cell. Microbiol. 13, 1107–1113 (2011).
Paragas, N. et al. α-Intercalated cells defend the urinary system from bacterial infection. J. Clin. Invest. 124, 2963–2976 (2014).
Pichon, C. et al. Uropathogenic Escherichia coli AL511 requires flagellum to enter renal collecting duct cells. Cell. Microbiol. 11, 616–628 (2009).
Roberts, J. A. et al. The Gal(α1–4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc. Natl Acad. Sci. USA 91, 11889–11893 (1994).
Chassin, C. et al. TLR4 facilitates translocation of bacteria across renal collecting duct cells. J. Am. Soc. Nephrol. 19, 2364–2374 (2008).
Anderson, G. G., Goller, C. C., Justice, S., Hultgren, S. J. & Seed, P. C. Polysaccharide capsule and sialic acid-mediated regulation promote biofilm-like intracellular bacterial communities during cystitis. Infect. Immun. 78, 963–975 (2010).
Smith, Y. C., Rasmussen, S. B., Grande, K. K., Conran, R. M. & O'Brien, A. D. Hemolysin of uropathogenic Escherichia coli evokes extensive shedding of the uroepithelium and hemorrhage in bladder tissue within the first 24 hours after intraurethral inoculation of mice. Infect. Immun. 76, 2978–2990 (2008).
Rippere-Lampe, K. E., O'Brien, A. D., Conran, R. & Lockman, H. A. Mutation of the gene encoding cytotoxic necrotizing factor type 1 (cnf(1)) attenuates the virulence of uropathogenic Escherichia coli. Infect. Immun. 69, 3954–3964 (2001).
Ashkar, A. A., Mossman, K. L., Coombes, B. K., Gyles, C. L. & Mackenzie, R. FimH adhesin of type 1 fimbriae is a potent inducer of innate antimicrobial responses which requires TLR4 and type 1 interferon signalling. PLoS Pathog. 4, e1000233 (2008).
Ragnarsdottir, B. et al. Reduced toll-like receptor 4 expression in children with asymptomatic bacteriuria. J. Infect. Dis. 196, 475–484 (2007).
Weichhart, T., Haidinger, M., Horl, W. H. & Saemann, M. D. Current concepts of molecular defence mechanisms operative during urinary tract infection. Eur. J. Clin. Invest. 38 (Suppl. 2), 29–38 (2008).
Hains, D. S. et al Carbonic anhydrase 2 deficiency leads to increased pyelonephritis susceptibility. Am. J. Physiol. Renal Physiol. 307, F869–F880 (2014).
Gauer, S. et al. IL-18 is expressed in the intercalated cell of human kidney. Kidney Int. 72, 1081–1087 (2007).
Ali, A. S., Townes, C. L., Hall, J. & Pickard, R. S. Maintaining a sterile urinary tract: the role of antimicrobial peptides. J. Urol. 182, 21–28 (2009).
Bevins, C. L. & Salzman, N. H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 9, 356–368 (2011).
Martinez, J. J., Mulvey, M. A., Schilling, J. D., Pinkner, J. S. & Hultgren, S. J. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 19, 2803–2812 (2000).
Schilling, J. D., Mulvey, M. A., Vincent, C. D., Lorenz, R. G. & Hultgren, S. J. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J. Immunol. 166, 1148–1155 (2001).
Bens, M. et al. Flagellin/TLR5 signalling activates renal collecting duct cells and facilitates invasion and cellular translocation of uropathogenic Escherichia coli. Cell. Microbiol. 16, 1503–1517 (2014).
Andersen-Nissen, E. et al. Cutting edge: Tlr5-/- mice are more susceptible to Escherichia coli urinary tract infection. J. Immunol. 178, 4717–4720 (2007).
Zhang, D. et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 303, 1522–1526 (2004).
Wang, G., Li, X. & Wang, Z. APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 37, D933–D937 (2009).
Ganz, T. The role of antimicrobial peptides in innate immunity. Integr. Comp. Biol. 43, 300–304 (2003).
Walch, M. et al. Cytotoxic cells kill intracellular bacteria through granulysin-mediated delivery of granzymes. Cell 157, 1309–1323 (2014).
Kudryashova, E. et al. Human defensins facilitate local unfolding of thermodynamically unstable regions of bacterial protein toxins. Immunity 41, 709–721 (2014).
Rathinakumar, R., Walkenhorst, W. F. & Wimley, W. C. Broad-spectrum antimicrobial peptides by rational combinatorial design and high-throughput screening: the importance of interfacial activity. J. Am. Chem. Soc. 131, 7609–7617 (2009).
Almeida, P. F. & Pokorny, A. Mechanisms of antimicrobial, cytolytic, and cell-penetrating peptides: from kinetics to thermodynamics. Biochemistry 48, 8083–8093 (2009).
Wang, H. et al. Contribution of structural domains to the activity of ribonuclease 7 against uropathogenic bacteria. Antimicrob. Agents Chemother. 57, 766–774 (2013).
Valore, E. V. et al. Human β-defensin-1: an antimicrobial peptide of urogenital tissues. J. Clin. Invest. 101, 1633–1642 (1998).
Abou Alaiwa, M. H. et al. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc. Natl Acad. Sci. USA 111, 18703–18708 (2014).
Schroeder, B. O. et al Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature 469, 419–423 (2011).
Guilhelmelli, F. et al. Antibiotic development challenges: the various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front. Microbiol. 4, 353 (2013).
Kai-Larsen, Y. et al. Uropathogenic Escherichia coli modulates immune responses and its curli fimbriae interact with the antimicrobial peptide LL-37. PLoS Pathog. 6, e1001010 (2010).
Yang, D. et al. Defensin participation in innate and adaptive immunity. Curr. Pharm. Des. 13, 3131–3139 (2007).
Neumann, A. et al. The antimicrobial peptide LL-37 facilitates the formation of neutrophil extracellular traps. Biochem. J. 464, 3–11 (2014).
Kruger, P. et al. Neutrophils: between host defence, immune modulation, and tissue injury. PLoS Pathog. 11, e1004651 (2015).
Chromek, M. et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 12, 636–641 (2006).
Nielsen, K. L. et al. Role of urinary cathelicidin LL-37 and human β-defensin 1 in uncomplicated Escherichia coli urinary tract infections. Infect. Immun. 82, 1572–1578 (2014).
Chromek, M. The role of the antimicrobial peptide cathelicidin in renal diseases. Pediatr. Nephrol. http://dx.doi.org/10.1007/s00467-014-2895-3.
Danka, E. S. & Hunstad, D. A. Cathelicidin augments epithelial receptivity and pathogenesis in experimental Escherichia coli cystitis. J. Infect. Dis. 211, 1164–1173 (2015).
Oottamasathien, S. et al. A murine model of inflammatory bladder disease: cathelicidin peptide induced bladder inflammation and treatment with sulfated polysaccharides. J. Urol. 186, 1684–1692 (2011).
Johansson, J., Gudmundsson, G. H., Rottenberg, M. E., Berndt, K. D. & Agerberth, B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 273, 3718–3724 (1998).
Lehrer, R. I., Lichtenstein, A. K. & Ganz, T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu. Rev. Immunol. 11, 105–128 (1993).
Liu, L., Zhao, C., Heng, H. H. & Ganz, T. The human β-defensin-1 and α-defensins are encoded by adjacent genes: two peptide families with differing disulfide topology share a common ancestry. Genomics 43, 316–320 (1997).
Ganz, T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3, 710–720 (2003).
Ihi, T., Nakazato, M., Mukae, H. & Matsukura, S. Elevated concentrations of human neutrophil peptides in plasma, blood, and body fluids from patients with infections. Clin. Infect. Dis. 25, 1134–1140 (1997).
Tikhonov, I., Rebenok, A. & Chyzh, A. A study of interleukin-8 and defensins in urine and plasma of patients with pyelonephritis and glomerulonephritis. Nephrol. Dial. Transplant. 12, 2557–2561 (1997).
Porter, E. et al. Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell-neutrophil interactions. Infect. Immun. 73, 4823–4833 (2005).
Quayle, A. J. et al. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am. J. Pathol. 152, 1247–1258 (1998).
Wang, A. P. et al. Antibacterial activity and mechanism of recombinant human α defensin 5 against clinical antibiotic-resistant strains. African J. Microbiol. Res. 4, 626–633 (2010).
Spencer, J. D. et al. Human α defensin 5 expression in the human kidney and urinary tract. PLoS ONE 7, e31712 (2012).
Porter, E. M. et al. Isolation of human intestinal defensins from ileal neobladder urine. FEBS Lett. 434, 272–276 (1998).
Townes, C. L., Ali, A., Robson, W., Pickard, R. & Hall, J. Tolerance of bacteriuria after urinary diversion is linked to antimicrobial peptide activity. Urology 77, 509.e1–509.e8 (2011).
Schutte, B. C. et al. Discovery of five conserved β-defensin gene clusters using a computational search strategy. Proc. Natl Acad. Sci. USA 99, 2129–2133 (2002).
Lehmann, J. et al. Expression of human β-defensins 1 and 2 in kidneys with chronic bacterial infection. BMC Infect. Dis. 2, 20 (2002).
Zucht, H. D. et al. Human β-defensin-1: a urinary peptide present in variant molecular forms and its putative functional implication. Eur. J. Med. Res. 3, 315–323 (1998).
Hiratsuka, T. et al. Structural analysis of human β-defensin-1 and its significance in urinary tract infection. Nephron 85, 34–40 (2000).
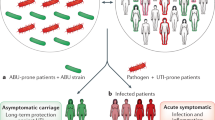
Nienhouse, V. et al. Interplay between bladder microbiota and urinary antimicrobial peptides: mechanisms for human urinary tract infection risk and symptom severity. PLoS ONE 9, e114185 (2014).
Kraemer, B. F. et al. Novel anti-bacterial activities of β-defensin 1 in human platelets: suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 7, e1002355 (2011).
Kandaswamy, K. et al. Focal targeting by human β-defensin 2 disrupts localized virulence factor assembly sites in Enterococcus faecalis. Proc. Natl Acad. Sci. USA 110, 20230–20235 (2013).
Rohrl, J., Yang, D., Oppenheim, J. J. & Hehlgans, T. Human β-defensin 2 and 3 and their mouse orthologs induce chemotaxis through interaction with CCR2. J. Immunol. 184, 6688–6694 (2010).
Zhao, J., Wang, Z., Chen, X., Wang, J. & Li, J. Effects of intravesical liposome-mediated human β-defensin-2 gene transfection in a mouse urinary tract infection model. Microbiol. Immunol. 55, 217–223 (2011).
Huttner, K. M., Kozak, C. A. & Bevins, C. L. The mouse genome encodes a single homolog of the antimicrobial peptide human β-defensin 1. FEBS Lett. 413, 45–49 (1997).
Bals, R., Goldman, M. J. & Wilson, J. M. Mouse β-defensin 1 is a salt-sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract. Infect. Immun. 66, 1225–1232 (1998).
Morrison, G. M. et al. Mouse β defensin-1 is a functional homolog of human β defensin-1. Mamm. Genome 9, 453–457 (1998).
Becknell, B. et al. Expression and antimicrobial function of β-defensin 1 in the lower urinary tract. PLoS ONE 8, e77714 (2013).
Morrison, G., Kilanowski, F., Davidson, D. & Dorin, J. Characterization of the mouse β defensin 1, Defb1, mutant mouse model. Infect. Immun. 70, 3053–3060 (2002).
Biragyn, A. et al. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science 298, 1025–1029 (2002).
Zhou, Y. S. et al. Partial deletion of chromosome 8 β-defensin cluster confers sperm dysfunction and infertility in male mice. PLoS Genet. 9, e1003826 (2013).
Navid, F. et al. Induction of regulatory T cells by a murine β-defensin. J. Immunol. 188, 735–743 (2012).
Rosenberg, H. F. RNase A ribonucleases and host defense: an evolving story. J. Leukoc. Biol. 83, 1079–1087 (2008).
Simanski, M., Dressel, S., Glaser, R. & Harder, J. RNase 7 protects healthy skin from Staphylococcus aureus colonization. J. Invest. Dermatol. 130, 2836–2838 (2010).
Becknell, B. et al. Ribonucleases 6 and 7 have antimicrobial function in the human and murine urinary tract. Kidney Int. 87, 151–161 (2014).
Spencer, J. D. et al. Ribonuclease 7 is a potent antimicrobial peptide within the human urinary tract. Kidney Int. 80, 174–180 (2011).
Spencer, J. D. et al. Ribonuclease 7, an antimicrobial peptide upregulated during infection, contributes to microbial defense of the human urinary tract. Kidney Int. 83, 615–625. (2013).
Boix, E. & Nogues, M. V. Mammalian antimicrobial proteins and peptides: overview on the RNase A superfamily members involved in innate host defence. Mol. Biosyst. 3, 317–335 (2007).
Huang, Y. C. et al. The flexible and clustered lysine residues of human ribonuclease 7 are critical for membrane permeability and antimicrobial activity. J. Biol. Chem. 282, 4626–4633 (2007).
Harder, J. & Schroder, J. M. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J. Biol. Chem. 277, 46779–46784 (2002).
Wang, H. et al. Contribution of structural domains to ribonuclease 7's activity against uropathogenic bacteria. Antimicrob. Agents Chemother. 57, 766–774 (2013).
Spencer, J. D. et al. An endogenous ribonuclease inhibitor regulates the antimicrobial activity of ribonuclease 7 in the human urinary tract. Kidney Int. 85, 1179–1191 (2013).
Abtin, A. et al. Degradation by stratum corneum proteases prevents endogenous RNase inhibitor from blocking antimicrobial activities of RNase 5 and RNase 7. J. Invest. Dermatol. 129, 2193–2201 (2009).
Blazquez, M., Fominaya, J. M. & Hofsteenge, J. Oxidation of sulfhydryl groups of ribonuclease inhibitor in epithelial cells is sufficient for its intracellular degradation. J. Biol. Chem. 271, 18638–18642 (1996).
Zasloff, M. The antibacterial shield of the human urinary tract. Kidney Int. 83, 548–550 (2013).
Hagan, E. C., Lloyd, A. L., Rasko, D. A., Faerber, G. J. & Mobley, H. L. Escherichia coli global gene expression in urine from women with urinary tract infection. PLoS Pathog. 6, e1001187 (2010).
Nemeth, E. et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–2093 (2004).
Park, C. H., Valore, E. V., Waring, A. J. & Ganz, T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J. Biol. Chem. 276, 7806–7810 (2001).
Moulouel, B. et al. Hepcidin regulates intrarenal iron handling at the distal nephron. Kidney Int. 84, 756–766 (2013).
Bellamy, W., Takase, M., Wakabayashi, H., Kawase, K. & Tomita, M. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J. Appl. Bacteriol. 73, 472–479 (1992).
Abrink, M., Larsson, E., Gobl, A. & Hellman, L. Expression of lactoferrin in the kidney: implications for innate immunity and iron metabolism. Kidney Int. 57, 2004–2010 (2000).
Paragas, N. et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat. Med. 17, 216–222 (2011).
Berger, T. et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc. Natl Acad. Sci. USA 103, 1834–1839 (2006).
Flo, T. H. et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432, 917–921 (2004).
Holmes, M. A., Paulsene, W., Jide, X., Ratledge, C. & Strong, R. K. Siderocalin (Lcn 2) also binds carboxymycobactins, potentially defending against mycobacterial infections through iron sequestration. Structure 13, 29–41 (2005).
Steigedal, M. et al. Lipocalin 2 imparts selective pressure on bacterial growth in the bladder and is elevated in women with urinary tract infection. J. Immunol. 193, 6081–6089 (2014).
Corbin, B. D. et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319, 962–965 (2008).
van Zoelen, M. A. et al. Expression and role of myeloid-related protein-14 in clinical and experimental sepsis. Am. J. Respir. Crit. Care Med. 180, 1098–1106 (2009).
Dessing, M. C. et al. S100A8/A9 is not involved in host defense against murine urinary tract infection. PLoS ONE 5, e13394 (2010).
Jaillon, S. et al. The humoral pattern recognition molecule PTX3 is a key component of innate immunity against urinary tract infection. Immunity 40, 621–632 (2014).
Mayrer, A. R., Miniter, P. & Andriole, V. T. Immunopathogenesis of chronic pyelonephritis. Am. J. Med. 75, 59–70 (1983).
Kokot, F. & Dulawa, J. Tamm-Horsfall protein updated. Nephron 85, 97–102 (2000).
Darisipudi, M. N. et al. Uromodulin triggers IL-1β-dependent innate immunity via the NLRP3 inflammasome. J. Am. Soc. Nephrol. 23, 1783–1789 (2012).
Muchmore, A. V. & Decker, J. M. Uromodulin: a unique 85-kilodalton immunosuppressive glycoprotein isolated from urine of pregnant women. Science 229, 479–481 (1985).
Hawthorn, L. A., Bruce, A. W. & Reid, G. Ability of uropathogens to bind to Tamm Horsfall protein-coated renal tubular cells. Urol. Res. 19, 301–304 (1991).
Bates, J. M. et al. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int. 65, 791–797 (2004).
Pak, J., Pu, Y., Zhang, Z. T., Hasty, D. L. & Wu, X. R. Tamm-Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. coli from binding to uroplakin Ia and Ib receptors. J. Biol. Chem. 276, 9924–9930 (2001).
Saemann, M. D. et al. Tamm-Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4-dependent mechanism. J. Clin. Invest. 115, 468–475 (2005).
Peschel, A. & Sahl, H. G. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 4, 529–536 (2006).
Haversen, L. A. et al. Human lactoferrin and peptides derived from a surface-exposed helical region reduce experimental Escherichia coli urinary tract infection in mice. Infect. Immun. 68, 5816–5823 (2000).
Hertting, O. et al. Vitamin D induction of the human antimicrobial Peptide cathelicidin in the urinary bladder. PLoS ONE 5, e15580 (2010).
Luthje, P. et al. Estrogen supports urothelial defense mechanisms. Sci. Transl. Med. 5, 190ra80 (2013).
American Academy of Pediatrics. Committee on Quality Improvement. Subcommittee on Urinary Tract Infection. Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics 103, 843–852 (1999).
Wolfe, A. J. et al. Evidence of uncultivated bacteria in the adult female bladder. J. Clin. Microbiol. 50, 1376–1383 (2012).
Chen, Q. X. et al. Genomic variations within DEFB1 are associated with the susceptibility to and the fatal outcome of severe sepsis in Chinese Han population. Genes Immun. 8, 439–443 (2007).
Hardwick, R. J. et al. β-defensin genomic copy number is associated with HIV load and immune reconstitution in sub-saharan Africans. J. Infect. Dis. 206, 1012–1019 (2012).
Acknowledgements
J.D.S.'s work is supported by an NIH Grant (NIDDK) K08 DK094970. B.B.'s work is supported by an NIH Grant (NIDDK) K08 DK102594.01A1.
Author information
Authors and Affiliations
Contributions
J.D.S. and B.B. researched data for the article and provided substantial contributions to discussions of its content. All authors wrote the article and reviewed/edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Becknell, B., Schwaderer, A., Hains, D. et al. Amplifying renal immunity: the role of antimicrobial peptides in pyelonephritis. Nat Rev Nephrol 11, 642–655 (2015). https://doi.org/10.1038/nrneph.2015.105
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2015.105
This article is cited by
-
Concentration of novel urinary tract infection biomarkers in neonates
Scientific Reports (2024)
-
Intercalated cell function, kidney innate immunity, and urinary tract infections
Pflügers Archiv - European Journal of Physiology (2024)
-
Uropathogen and host responses in pyelonephritis
Nature Reviews Nephrology (2023)
-
Host and Pathogen-Directed Therapies against Microbial Infections Using Exosome- and Antimicrobial Peptide-derived Stem Cells with a Special look at Pulmonary Infections and Sepsis
Stem Cell Reviews and Reports (2023)
-
The urothelium: a multi-faceted barrier against a harsh environment
Mucosal Immunology (2022)