Abstract
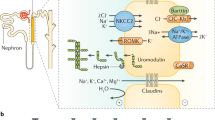
Alagille syndrome is an autosomal dominant disorder with variable multisystem organ involvement that is caused by mutations in one of two genes in the Notch signalling pathway, JAG1 or NOTCH2. Alagille syndrome is characterized by bile duct paucity, along with at least three of the following features: cholestasis, cardiac defects, skeletal abnormalities, ocular abnormalities and characteristic facies. However, the clinical features of Alagille syndrome are highly variable, and children or adults may also present with predominantly renal findings and little or no hepatic involvement. Renal involvement occurs in 40% of JAG1-mutation-positive individuals. Renal insufficiency is common and has been specifically reported in children with Alagille syndrome who have end-stage liver disease. The role of NOTCH2 and JAG1 in formation of proximal nephron structures and podocytes might explain the observed phenotypes of renal dysplasia and proteinuria in patients with Alagille syndrome, and renal tubular acidosis may be the result of JAG1 expression in the collecting ducts. Renal vascular hypertension in patients with Alagille syndrome is explained by the widespread vasculopathy and the role of Notch signalling in vascular development. Increased awareness of Alagille syndrome amongst nephrologists may lead to more diagnoses of Alagille syndrome in patients with apparently isolated renal disease.
Key Points
-
Alagille syndrome is an autosomal dominant disorder with variable multisystem organ involvement
-
Classic features of Alagille syndrome include cholestatic liver disease, congenital cardiac disease (peripheral pulmonary stenosis), vasculopathy, renal pathology, skeletal features (butterfly vertebrae), ophthalmological involvement (posterior embryotoxon) and characteristic facies
-
Alagille syndrome can present at any age, even in adulthood, with predominantly renal and little or no hepatic involvement
-
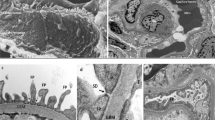
Renal findings in Alagille syndrome include renal dysplasia (with or without cysts), renal tubular acidosis, vesicoureteral reflux, proteinuria and hypertension (with midaortic syndrome and/or visceral artery stenosis)
-
Renal pathology and vasculopathy have been recognized to be recurrent features of Alagille syndrome, which should be included in diagnostic criteria
-
Renal phenotypes in Alagille syndrome can be explained by the presence of mutations in the Notch signalling pathway genes, NOTCH2 or JAG1, during kidney development
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Alagille, D. et al. Syndromic paucity of interlobular bile ducts (Alagille syndrome or arteriohepatic dysplasia): review of 80 cases. J. Pediatr. 2, 195–200 (1987).
Crosnier, C., Lykavieris, P., Meunier-Rotival, M. & Hadchouel, M. Alagille syndrome. The widening spectrum of arteriohepatic dysplasia. Clin. Liver Dis. 4, 765–778 (2000).
Kamath, B. M., Bason, L., Piccoli, D. A., Krantz, I. D. & Spinner, N. B. Consequences of JAG1 mutations. J. Med. Genet. 40, 891–895 (2003).
Emerick, K. M. et al. Features of Alagille syndrome in 92 patients: frequency and relation to prognosis. Hepatology 29, 822–829 (1999).
Kamath, B. M. et al. Renal anomalies in Alagille syndrome. Am. J. Med. Genet. 1, 185–189 (2012).
Kamath, B. M. et al. Vascular anomalies in Alagille syndrome: a significant cause of morbidity and mortality. Circulation 109, 1354–1358 (2004).
Danks D. M. et al. Studies of the aetiology of neonatal hepatitis and biliary atresia. Arch. Dis. Child. 5, 360–367 (1977).
Bauer, R. C. et al. Jagged1 (JAG1) mutations in patients with tetralogy of Fallot or pulmonic stenosis. Hum. Mutat. 31, 594–601 (2010).
Harendza, S. et al. Renal failure and hypertension in Alagille syndrome with a novel JAG1 mutation. J. Nephrol 18, 312–317 (2005).
Jacquet, A. et al. Alagille syndrome in adult patients: it is never too late. Am. J. Kidney Dis. 49, 705–709 (2007).
Li, L. et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat. Genet. 16, 243–251 (1997).
McDaniell, R. et al. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am. J. Hum. Genet. 79, 169–173 (2006).
Oda, T. et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat. Genet. 16, 235–242 (1997).
Warthen, D. M. et al. Jagged1 (JAG1) mutations in Alagille syndrome: increasing the mutation detection rate. Hum. Mutat. 27, 436–443 (2006).
Kamath, B. M., Loomes, K. M. & Piccoli, D. A. Medical management of Alagille syndrome. Pediatr. Gastroenterol. Nutr. 6, 580–586 (2010).
Emerick, K. M. & Whitington, P.F. Partial external biliary diversion for intractable pruritus and xanthomas in Alagille syndrome. Hepatology 6, 1501–1506 (2002).
Kamath, B. M., Schwarz, K. B. & Hadzic, N. Alagille syndrome and liver transplantation. J. Pediatr. Gastroenterol. Nutr. 50, 11–15 (2010).
McElhinney, D. B. et al. Analysis of cardiovascular phenotype and genotype-phenotype correlation in individuals with a JAG1 mutation and/or Alagille syndrome. Circulation 106, 2567–2574 (2002).
Emerick, K. M. et al. Intracranial vascular abnormalities in patients with Alagille syndrome. J. Pediatr. Gastroenterol. Nutr. 41, 99–107 (2005).
Bales, C. B. et al. Pathologic lower extremity fractures in children with Alagille syndrome. J. Pediatr. Gastroenterol. Nutr. 51, 66–70 (2010).
Kamath, B. M. et al. Facial features in Alagille syndrome: specific or cholestasis facies? Am. J. Med. Genet. 112, 163–170 (2002).
Rennie, C. A. et al. The prevalence and associated features of posterior embryotoxon in the general ophthalmic clinic. Eye (Lond.) 19, 396–399 (2005).
Benoit, G. et al. Mesangiolipidosis in Alagille syndrome—relationship with apolipoprotein A-I. Nephrol. Dial. Transplant. 22, 2072–2075 (2007).
Berard, E. et al. Renovascular hypertension and vascular anomalies in Alagille syndrome. Pediatr. Nephrol. 12, 121–124 (1998).
Bourdeaut, F. et al. Alagille syndrome and nephroblastoma: unusual coincidence of two rare disorders. Pediatr. Blood Cancer 50, 908–911 (2008).
Chung-Park, M. et al. Renal lipidosis associated with arteriohepatic dysplasia (Alagille's syndrome). Clin. Nephrol. 18, 314–320 (1982).
Davis, J., Griffiths, R., Larkin, K., Rozansky, D. & Troxell, M. Glomerular basement membrane lipidosis in Alagille syndrome. Pediatr. Nephrol. 25, 1181–1184 (2010).
Devriendt, K. et al. Paucity of intrahepatic bile ducts, solitary kidney and atrophic pancreas with diabetes mellitus: atypical Alagille syndrome? Eur. J. Pediatr. 155, 87–90 (1996).
Hirai, H. et al. Successful stenting for renal artery stenosis in a patient with Alagille syndrome. Pediatr. Nephrol. 20, 831–833 (2005).
Martin, S. R., Garel, L. & Alvarez, F. Alagille's syndrome associated with cystic renal disease. Arch. Dis. Child. 74, 232–235 (1996).
Pombo, F., Isla, C., Gayol, A. & Bargiela, A. Aortic calcification and renal cysts demonstrated by CT in a teenager with Alagille syndrome. Pediatr. Radiol. 25, 314–315 (1995).
Quiros-Tejeira, R. E. et al. Variable morbidity in alagille syndrome: a review of 43 cases. J. Pediatr. Gastroenterol. Nutr. 29, 431–437 (1999).
Salem, J. E., Bruguiere, E., Iserin, L., Guiochon-Mantel, A. & Plouin, P. F. Hypertension and aortorenal disease in Alagille syndrome. J. Hypertens. 30, 1300–1306 (2012).
Schonck, M., Hoorntje, S. & van Hooff, J. Renal transplantation in Alagille syndrome. Nephrol. Dial. Transplant. 13, 197–199 (1998).
Schwarzenberg, S. J., Grothe, R. M., Sharp, H. L., Snover, D. C. & Freese, D. Long-term complications of arteriohepatic dysplasia. Am. J. Med. 93, 171–176 (1992).
Shrivastava, R. et al. An unusual cause of hypertension and renal failure: a case series of a family with Alagille syndrome. Nephrol. Dial. Transplant. 25, 1501–1506 (2010).
Subramaniam, P. et al. Diagnosis of Alagille syndrome—25 years of experience at King's College Hospital. J. Pediatr. Gastroenterol. Nutr. 52, 84–89 (2011).
Tolia, V., Dubois, R. S., Watts, F. B., Jr & Perrin, E. Renal abnormalities in paucity of interlobular bile ducts. J. Pediatr. Gastroenterol. Nutr. 6, 971–976 (1987).
Wang, J. S. et al. Clinical and pathological characteristics of Alagille syndrome in Chinese children. World J. Pediatr. 4, 283–288 (2008).
Wolfish, N. M. & Shanon, A. Nephropathy in arteriohepatic dysplasia (Alagille's syndrome). Child. Nephrol. Urol. 9, 169–172 (1988).
Yucel, H., Hoorntje, S. J. & Bravenboer, B. Renal abnormalities in a family with Alagille syndrome. Neth. J. Med. 68, 38–39 (2010).
Habib, R. et al. Glomerular mesangiolipidosis in Alagille syndrome (arteriohepatic dysplasia). Pediatr. Nephrol. 1, 455–464 (1987).
Kamath, B. M. et al. Outcomes of liver transplantation for patients with Alagille syndrome: the studies of pediatric liver transplantation experience. Liver Transpl. 8, 940–948 (2012).
Kamath, B. M. et al. Facial features in Alagille syndrome: specific or cholestasis facies? Am. J. Med. Genet. 112, 163–170 (2002).
Crosnier, C. et al. Mutations in JAGGED1 gene are predominantly sporadic in Alagille syndrome. Gastroenterology 116, 1141–1148 (1999).
Spinner, N. B. et al. Jagged1 mutations in Alagille syndrome. Hum. Mutat. 17, 18–33 (2001).
Yerushalmi, B., Sokol, R. J., Narkewicz, M. R., Smith, D. & Karrer, F. M. Use of rifampin for severe pruritus in children with chronic cholestasis. J. Pediatr. Gastroenterol. Nutr. 29, 442–447 (1999).
Kamath, B. M. et al. NOTCH2 mutations in Alagille syndrome. J. Med. Genet. 2, 138–144 (2012).
Isidor, B. et al. Serpentine fibula-polycystic kidney syndrome caused by truncating mutations in NOTCH2. Hum. Mutat. 32, 1239–1242 (2011).
Simpson, M. A. et al. Mutations in NOTCH2 cause Hajdu-Cheney syndrome, a disorder of severe and progressive bone loss. Nat. Genet. 43, 303–305 (2011).
Gray, M. J. et al. Serpentine fibula polycystic kidney syndrome is part of the phenotypic spectrum of Hajdu-Cheney syndrome. Eur. J. Hum. Genet. 20, 122–124 (2012).
Gridley, T. Notch signaling during vascular development. Proc. Natl Acad. Sci. USA 98, 5377–5378 (2001).
Kopan, R. Notch signaling. Cold Spring Harb. Perspect. Biol. 4, 1–4 (2012).
Crosnier, C. et al. JAGGED1 gene expression during human embryogenesis elucidates the wide phenotypic spectrum of Alagille syndrome. Hepatology 32, 574–581 (2000).
McCright, B., Lozier, J. & Gridley, T. A mouse model of Alagille syndrome: notch2 as a genetic modifier of Jag1 haploinsufficiency. Development 129, 1075–1082 (2002).
McCright, B. Notch signaling in kidney development. Curr. Opin. Nephrol. Hypertens. 12, 5–10 (2003).
Sirin, Y. & Susztak, K. Notch in the kidney: development and disease. J. Pathol. 226, 394–403 (2012).
Cheng, H. T. & Kopan, R. The role of Notch signaling in specification of podocyte and proximal tubules within the developing mouse kidney. Kidney Int. 68, 1951–1952 (2005).
McCright, B. et al. Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development 128, 491–502 (2001).
Weinmaster, G., Roberts, V. J. & Lemke, G. Notch2: a second mammalian Notch gene. Development 116, 931–941 (1992).
Cheng, H. T. et al. Gamma-secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development 130, 5031–5042 (2003).
Krebs, L. T. et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 14, 1343–1352 (2000).
Uyttendaele, H., Ho, J., Rossant, J. & Kitajewski, J. Vascular patterning defects associated with expression of activated Notch4 in embryonic endothelium. Proc. Natl Acad. Sci. USA 98, 5643–5648 (2001).
Xue, Y. et al. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum. Mol. Genet. 8, 723–730 (1999).
Cheng, H. T. et al. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development 134, 801–811 (2007).
Bonegio, R. G., Beck, L. H., Kahlon, R. K., Lu, W. & Salant, D. J. The fate of Notch-deficient nephrogenic progenitor cells during metanephric kidney development. Kidney Int. 79, 1099–1112 (2011).
Fujimura, S., Jiang, Q., Kobayashi, C. & Nishinakamura, R. Notch2 activation in the embryonic kidney depletes nephron progenitors. J. Am. Soc. Nephrol. 21, 803–810 (2010).
Si-Tayeb, K., Lemaigre F. P. & Duncan, S. Organogenesis and development of the liver. Development 18, 175–189 (2010).
Kodama, Y., Hijikata, M., Kageyama, R., Shimotohno, K. & Chiba, T. The role of Notch signaling in the development of intrahepatic bile ducts. Gastroenterology 127, 1775–1786 (2004).
Hofmann, J. J. et al. Jagged in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development 137, 4061–4072 (2010).
Walsh, D. W. et al. Co-regulation of Gremlin and Notch signalling in diabetic nephropathy. Biochim. Biophys. Acta 1782, 10–21 (2008).
Murea, M. et al. Expression of Notch pathway proteins correlates with albuminuria, glomerulosclerosis and renal function. Kidney Int. 78, 514–522 (2010).
Kobayashi, T. et al. Expression and function of the Delta-1/Notch-2/Hes-1 pathway during experimental acute kidney injury. Kidney Int. 73, 1240–1250 (2008).
Oestrich, A. E., Sokol, R. J., Suchy, F. J. & Heubi, J. E. Renal abnormalities in arteriohepatic dysplasia and nonsyndromic intrahepatic biliary hypoplasia. Ann. Radiol. (Paris) 26, 203–209 (1983).
Hyams, J. S., Berman, M. M & Davis, B. H. Tubulointerstitial nephropathy associated with arteriohepatic dysplasia. Gastroenterology 85, 430–434 (1983).
Acknowledgements
The authors' research work is supported by grants from the Canadian Institutes of Health Research, Kidney Foundation of Canada and the Canada Research Chairs Program (to N. D. Rosenblum) and by funding from the National Institute of Diabetes, Digestive and Kidney Diseases via the Childhood Liver Disease and Research Consortium: grant number U54DK078377 to B. M. Kamath and N. B. Spinner, and grant number DK081702 to N. B. Spinner.
Author information
Authors and Affiliations
Contributions
B. M. Kamath researched the data for the article. B. M. Kamath, N. B. Spinner and N. D. Rosenblum contributed to writing, substantial contributions to discussions of the content, and review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kamath, B., Spinner, N. & Rosenblum, N. Renal involvement and the role of Notch signalling in Alagille syndrome. Nat Rev Nephrol 9, 409–418 (2013). https://doi.org/10.1038/nrneph.2013.102
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2013.102
This article is cited by
-
Notch signaling pathway: architecture, disease, and therapeutics
Signal Transduction and Targeted Therapy (2022)
-
Kidney disease in children with heart or liver transplant
Pediatric Nephrology (2021)