Abstract
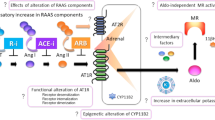
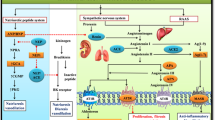
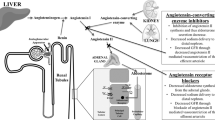
The renin–angiotensin–aldosterone system (RAAS) was initially thought to be fairly simple. However, this idea has been challenged following the development of RAAS blockers, including renin inhibitors, angiotensin-converting-enzyme (ACE) inhibitors, type 1 angiotensin II (AT1)-receptor blockers and mineralocorticoid-receptor antagonists. Consequently, new RAAS components and pathways that might contribute to the effectiveness of these drugs and/or their adverse effects have been identified. For example, an increase in renin levels during RAAS blockade might result in harmful effects via stimulation of the prorenin receptor (PRR), and prorenin—the inactive precursor of renin—might gain enzymatic activity on PRR binding. The increase in angiotensin II levels that occurs during AT1-receptor blockade might result in beneficial effects via stimulation of type 2 angiotensin II receptors. Moreover, angiotensin 1–7 levels increase during ACE inhibition and AT1-receptor blockade, resulting in Mas receptor activation and the induction of cardioprotective and renoprotective effects, including stimulation of tissue repair by stem cells. Finally, a role of angiotensin II in sodium and potassium handling in the distal nephron has been identified. This finding is likely to have important implications for understanding the effects of RAAS inhibition on whole body sodium and potassium balance.
Key Points
-
Interactions between renin, prorenin and the prorenin receptor (PRR) have not been confirmed in vivo and seem unlikely because of the low levels of renin and prorenin in blood
-
Given the role of the PRR in V-type proton ATPase integrity and Wnt signalling, renin–angiotensin–aldosterone system (RAAS)-independent functions of the PRR seems more likely than RAAS-dependent functions
-
Under certain pathological conditions, type 2 angiotensin II (AT2) receptors mimic type 1 angiotensin II (AT1) receptor function and exert detrimental effects including vasoconstriction and hypertrophy
-
Stimulating angiotensin 1–7 generation or using stable angiotensin 1–7 analogues to activate the Mas receptor is a promising new strategy to improve tissue repair by stem cells
-
Angiotensin II affects the activity of the main sodium and potassium transporters in the distal nephron: the sodium chloride cotransporter, epithelial sodium channel and renal outer medullary potassium channel
-
Synergistic actions of angiotensin II and aldosterone on sodium and potassium transport in the distal nephron help to explain the effects of RAAS inhibition on renal sodium and potassium excretion
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Klotz, S., Burkhoff, D., Garrelds, I. M., Boomsma, F. & Danser, A. H. J. The impact of left ventricular assist device-induced left ventricular unloading on the myocardial renin-angiotensin-aldosterone system: therapeutic consequences? Eur. Heart J. 30, 805–812 (2009).
van Kats, J. P. et al. Angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade prevent cardiac remodeling in pigs after myocardial infarction: role of tissue angiotensin II. Circulation 102, 1556–1563 (2000).
Ma, T. K., Kam, K. K., Yan, B. P. & Lam, Y. Y. Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: current status. Br. J. Pharmacol. 160, 1273–1292 (2010).
Verdonk, K., Danser, A. H. J. & van Esch, J. H. M. Angiotensin II type 2 receptor agonists: where should they be applied? Expert Opin. Investig. Drugs 21, 501–513 (2012).
van Esch, J. H. M. et al. AT2 receptor-mediated vasodilation in the mouse heart depends on AT1A receptor activation. Br. J. Pharmacol. 148, 452–458 (2006).
Santos, R. A. et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc. Natl Acad. Sci. USA 100, 8258–8263 (2003).
Danser, A. H. Novel drugs targeting hypertension: renin inhibitors. J. Cardiovasc. Pharmacol. 50, 105–111 (2007).
Nguyen, G. et al. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Invest. 109, 1417–1427 (2002).
Danser, A. H. J. The increase in renin during renin inhibition: does it result in harmful effects by the (pro)renin receptor? Hypertens. Res. 33, 4–10 (2010).
Batenburg, W. W., Jansen, P. M., van den Bogaerdt, A. J. & Danser, A. H. J. Angiotensin II-aldosterone interaction in human coronary microarteries involves GPR30, EGFR, and endothelial NO synthase. Cardiovasc. Res. 94, 136–143 (2012).
Chai, W. et al. Steroidogenesis vs. steroid uptake in the heart: do corticosteroids mediate effects via cardiac mineralocorticoid receptors? J. Hypertens. 28, 1044–1053 (2010).
Chai, W. et al. Nongenomic effects of aldosterone in the human heart. Interaction with angiotensin II. Hypertension 46, 701–706 (2005).
Batenburg, W. W. et al. Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor. J. Hypertens. 25, 2441–2453 (2007).
Lu, X., Danser, A. H. J. & Meima, M. E. HRP and prorenin: focus on the (pro)renin receptor and vacuolar H+-ATPase. Front. Biosci. (Schol. Ed.) 3, 1205–1215 (2011).
Krop, M. & Danser, A. H. J. Circulating versus tissue renin-angiotensin system: on the origin of (pro)renin. Curr. Hypertens. Rep. 10, 112–118 (2008).
Saris, J. J. et al. Cardiomyocytes bind and activate native human prorenin: role of soluble mannose 6-phosphate receptors. Hypertension 37, 710–715 (2001).
Feldt, S. et al. Prorenin and renin-induced extracellular signal-regulated kinase 1/2 activation in monocytes is not blocked by aliskiren or the handle-region peptide. Hypertension 51, 682–688 (2008).
Suzuki, F. et al. Human prorenin has “gate and handle” regions for its non-proteolytic activation. J. Biol. Chem. 278, 22217–22222 (2003).
Ichihara, A. et al. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J. Clin. Invest. 114, 1128–1135 (2004).
Ichihara, A. et al. Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor-deficient mice. J. Am. Soc. Nephrol. 17, 1950–1961 (2006).
Kaneshiro, Y. et al. Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J. Am. Soc. Nephrol. 18, 1789–1795 (2007).
Kaneshiro, Y. et al. Increased expression of cyclooxygenase-2 in the renal cortex of human prorenin receptor gene-transgenic rats. Kidney Int. 70, 641–646 (2006).
Jansen, P. M., Hofland, J., van den Meiracker, A. H., de Jong, F. H. & Danser, A. H. J. Renin and prorenin have no direct effect on aldosterone synthesis in the human adrenocortical cell lines H295R and HAC15. J. Renin Angiotensin Aldosterone Syst. 13, 360–366 (2012).
Huang, Y. et al. Renin increases mesangial cell transforming growth factor-β1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int. 69, 105–113 (2006).
Clavreul, N., Sansilvestri-Morel, P., Magard, D., Verbeuren, T. J. & Rupin, A. (Pro)renin promotes fibrosis gene expression in HEK cells through a Nox4-dependent mechanism. Am. J. Physiol. Renal Physiol. 300, F1310–F1318 (2011).
Sakoda, M. et al. Aliskiren inhibits intracellular angiotensin II levels without affecting (pro)renin receptor signals in human podocytes. Am. J. Hypertens. 23, 575–580 (2010).
Zhang, J., Gu, C., Noble, N. A., Border, W. A. & Huang, Y. Combining angiotensin II blockade and renin receptor inhibition results in enhanced antifibrotic effect in experimental nephritis. Am. J. Physiol. Renal Physiol. 301, F723–F732 (2011).
Melnyk, R. A., Tam, J., Boie, Y., Kennedy, B. P. & Percival, M. D. Renin and prorenin activate pathways implicated in organ damage in human mesangial cells independent of angiotensin II production. Am. J. Nephrol. 30, 232–243 (2009).
Cheng, H., Fan, X., Moeckel, G. W. & Harris, R. C. Podocyte COX-2 exacerbates diabetic nephropathy by increasing podocyte (pro)renin receptor expression. J. Am. Soc. Nephrol. 22, 1240–1251 (2011).
Huang, J. & Siragy, H. M. Glucose promotes the production of interleukine-1β and cyclooxygenase-2 in mesangial cells via enhanced (pro)renin receptor expression. Endocrinology 150, 5557–5565 (2009).
Siragy, H. M. & Huang, J. Renal (pro)renin receptor upregulation in diabetic rats through enhanced angiotensin AT1 receptor and NADPH oxidase activity. Exp. Physiol. 93, 709–714 (2008).
Matavelli, L., Huang, J. & Siragy, H. M. (Pro)renin receptor contributes to diabetic nephropathy by enhancing renal inflammation. Clin. Exp. Pharmacol. Physiol. 37, 277–282 (2010).
Feldman, D. L. et al. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mREN-2)-27 rats. Hypertension 52, 130–136 (2008).
Krebs, C. et al. Antihypertensive therapy upregulates renin and (pro)renin receptor in the clipped kidney of Goldblatt hypertensive rats. Kidney Int. 72, 725–730 (2007).
Hirose, T. et al. Increased expression of (pro)renin receptor in the remnant kidneys of 5/6 nephrectomized rats. Regul. Pept. 159, 93–99 (2010).
Takahashi, K. et al. Expression of (pro)renin receptor in human kidneys with end-stage kidney disease due to diabetic nephropathy. Peptides 31, 1405–1408 (2010).
Danser, A. H. J. et al. Determinants of interindividual variation of renin and prorenin concentrations: evidence for a sexual dimorphism of (pro)renin levels in humans. J. Hypertens. 16, 853–862 (1998).
Burcklé, C. A. et al. Elevated blood pressure and heart rate in human renin receptor transgenic rats. Hypertension 47, 552–556 (2006).
Batenburg, W. W. et al. Renin- and prorenin-induced effects in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor: does (pro)renin-(pro)renin receptor interaction actually occur? Hypertension 58, 1111–1119 (2011).
Peters, B. et al. Dose-dependent titration of prorenin and blood pressure in Cyp1a1ren-2 transgenic rats: absence of prorenin-induced glomerulosclerosis. J. Hypertens. 26, 102–109 (2008).
Campbell, D. J., Karam, H., Ménard, J., Bruneval, P. & Mullins, J. J. Prorenin contributes to angiotensin peptide formation in transgenic rats with rat prorenin expression targeted to the liver. Hypertension 54, 1248–1253 (2009).
Mercure, C., Prescott, G., Lacombe, M. J., Silversides, D. W. & Reudelhuber, T. L. Chronic increases in circulating prorenin are not associated with renal or cardiac pathologies. Hypertension 53, 1062–1069 (2009).
Campbell, D. J. et al. Activity assays and immunoassays for plasma renin and prorenin: information provided and precautions necessary for accurate measurement. Clin. Chem. 55, 867–877 (2009).
Richer-Giudicelli, C. et al. Haemodynamic effects of dual blockade of the renin-angiotensin system in spontaneously hypertensive rats: influence of salt. J. Hypertens. 22, 619–627 (2004).
de Boer, R. A. et al. Dual RAAS suppression: recent developments and implications in light of the ALTITUDE study. J. Renin Angiotensin Aldosterone Syst. 13, 409–412 (2012).
Advani, A. et al. The (pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension 54, 261–269 (2009).
Riediger, F. et al. Prorenin receptor is essential for podocyte autophagy and survival. J. Am. Soc. Nephrol. 22, 2193–2202 (2011).
Cruciat, C. M. et al. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327, 459–463 (2010).
van Esch, J. H. M. et al. Handle region peptide counteracts the beneficial effects of the renin inhibitor aliskiren in spontaneously hypertensive rats. Hypertension 57, 852–858 (2011).
Vázquez, E. et al. Angiotensin II-dependent induction of AT2 receptor expression after renal ablation. Am. J. Physiol. Renal Physiol. 288, F207–F213 (2005).
He, M. et al. Angiotensin II type 2 receptor mediated angiotensin II and high glucose induced decrease in renal prorenin/renin receptor expression. Mol. Cell Endocrinol. 315, 188–194 (2010).
Ruiz-Ortega, M. et al. Renal expression of angiotensin type 2 (AT2) receptors during kidney damage. Kidney Int. Suppl. S21–S26 (2003).
Ichiki, T. et al. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature 377, 748–750 (1995).
Hein, L., Barsh, G. S., Pratt, R. E., Dzau, V. J. & Kobilka, B. K. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature 377, 744–747 (1995).
Gross, V. et al. Inhibition of pressure natriuresis in mice lacking the AT2 receptor. Kidney Int. 57, 191–202 (2000).
Benndorf, R. A. et al. Angiotensin II type 2 receptor deficiency aggravates renal injury and reduces survival in chronic kidney disease in mice. Kidney Int. 75, 1039–1049 (2009).
Ma, J. et al. Accelerated fibrosis and collagen deposition develop in the renal interstitium of angiotensin type 2 receptor null mutant mice during ureteral obstruction. Kidney Int. 53, 937–944 (1998).
Tanaka, M. et al. Vascular response to angiotensin II is exaggerated through an upregulation of AT1 receptor in AT2 knockout mice. Biochem. Biophys. Res. Commun. 258, 194–198 (1999).
Padia, S. H., Howell, N. L., Siragy, H. M. & Carey, R. M. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin II type 1 receptor-blocked rat. Hypertension 47, 537–544 (2006).
van Esch, J. H. M., Oosterveer, C. R., Batenburg, W. W., van Veghel, R. & Danser, A. H. J. Effects of angiotensin II and its metabolites in the rat coronary vascular bed: is angiotensin III the preferred ligand of the angiotensin AT2 receptor? Eur. J. Pharmacol. 588, 286–293 (2008).
Padia, S. H. et al. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-mediated natriuresis in rats. Hypertension 51, 460–465 (2008).
Padia, S. H. et al. Intrarenal aminopeptidase N inhibition augments natriuretic responses to angiotensin III in angiotensin type 1 receptor-blocked rats. Hypertension 49, 625–630 (2007).
Ali, Q. & Hussain, T. AT2 receptor non-peptide agonist C21 promotes natriuresis in obese Zucker rats. Hypertens. Res. 35, 654–660 (2012).
Matavelli, L. C., Huang, J. & Siragy, H. M. Angiotensin AT2 receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension 57, 308–313 (2011).
Hilliard, L. M. et al. Sex-specific influence of angiotensin type 2 receptor stimulation on renal function: a novel therapeutic target for hypertension. Hypertension 59, 409–414 (2012).
Verdonk, K. et al. Compound 21 induces vasorelaxation via an endothelium and angiotensin II type 2 receptor-independent mechanism. Hypertension 60, 722–729 (2012).
Cao, Z. et al. Angiotensin type 2 receptor antagonism confers renal protection in a rat model of progressive renal injury. J. Am. Soc. Nephrol. 13, 1773–1787 (2002).
Esteban, V. et al. Angiotensin II, via AT1 and AT2 receptors and NF-κB pathway, regulates the inflammatory response in unilateral ureteral obstruction. J. Am. Soc. Nephrol. 15, 1514–1529 (2004).
Wolf, G. et al. Angiotensin II stimulates expression of the chemokine RANTES in rat glomerular endothelial cells. Role of the angiotensin type 2 receptor. J. Clin. Invest. 100, 1047–1058 (1997).
Siragy, H. M. & Carey, R. M. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension 33, 1237–1242 (1999).
Rehman, A. et al. Angiotensin type 2 receptor agonist compound 21 reduces vascular injury and myocardial fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension 59, 291–299 (2012).
Moltzer, E., Verkuil, A. V., van Veghel, R., Danser, A. H. J. & van Esch, J. H. M. Effects of angiotensin metabolites in the coronary vascular bed of the spontaneously hypertensive rat: loss of angiotensin II type 2 receptor-mediated vasodilation. Hypertension 55, 516–522 (2010).
You, D. et al. High blood pressure reduction reverses angiotensin II type 2 receptor-mediated vasoconstriction into vasodilation in spontaneously hypertensive rats. Circulation 111, 1006–1011 (2005).
Blood Pressure Lowering Treatment Trialists' Collaboration et al. Blood pressure-dependent and independent effects of agents that inhibit the renin–angiotensin system. J. Hypertens. 25, 951–958 (2007).
Vickers, C. et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 277, 14838–14843 (2002).
Iusuf, D., Henning, R. H., van Gilst, W. H. & Roks, A. J. Angiotensin-(1–7): pharmacological properties and pharmacotherapeutic perspectives. Eur. J. Pharmacol. 585, 303–312 (2008).
Ferreira, A. J. et al. Therapeutic implications of the vasoprotective axis of the renin-angiotensin system in cardiovascular diseases. Hypertension 55, 207–213 (2010).
Moon, J. Y. et al. Attenuating effect of angiotensin-(1–7) on angiotensin II-mediated NAD(P)H oxidase activation in type 2 diabetic nephropathy of KK-Ay/Ta mice. Am. J. Physiol. Renal Physiol. 300, F1271–F1282 (2011).
Benter, I. F. et al. Angiotensin-(1–7) blockade attenuates captopril- or hydralazine-induced cardiovascular protection in spontaneously hypertensive rats treated with NG-nitro-L-arginine methyl ester. J. Cardiovasc. Pharmacol. 57, 559–567 (2011).
Benter, I. F., Yousif, M. H., Anim, J. T., Cojocel, C. & Diz, D. I. Angiotensin-(1–7) prevents development of severe hypertension and end-organ damage in spontaneously hypertensive rats treated with L-NAME. Am. J. Physiol. Heart Circ. Physiol. 290, H684–H691 (2006).
Raffai, G., Durand, M. J. & Lombard, J. H. Acute and chronic angiotensin-(1–7) restores vasodilation and reduces oxidative stress in mesenteric arteries of salt-fed rats. Am. J. Physiol. Heart Circ. Physiol. 301, H1341–H1352 (2011).
Rabelo, L. A. et al. Ablation of angiotensin (1–7) receptor Mas in C57Bl/6 mice causes endothelial dysfunction. J. Am. Soc. Hypertens. 2, 418–424 (2008).
Sampaio, W. O., Henrique de Castro, C., Santos, R. A., Schiffrin, E. L. & Touyz, R. M. Angiotensin-(1–7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension 50, 1093–1098 (2007).
Wolf, G., Ziyadeh, F. N., Zahner, G. & Stahl, R. A. Angiotensin II-stimulated expression of transforming growth factor β in renal proximal tubular cells: attenuation after stable transfection with the c-mas oncogene. Kidney Int. 48, 1818–1827 (1995).
Zimpelmann, J. & Burns, K. D. Angiotensin-(1–7) activates growth-stimulatory pathways in human mesangial cells. Am. J. Physiol. Renal Physiol. 296, F337–F346 (2009).
Kostenis, E. et al. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation 111, 1806–1813 (2005).
Strauer, B. E. & Steinhoff, G. 10 years of intracoronary and intramyocardial bone marrow stem cell therapy of the heart: from the methodological origin to clinical practice. J. Am. Coll. Cardiol. 58, 1095–1104 (2011).
El-Ansary, M., Saadi, G. & Abd El-Hamid, S. Mesenchymal stem cells are a rescue approach for recovery of deteriorating kidney function. Nephrology (Carlton) 17, 650–657 (2012).
Perico, N. et al. Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility. Clin. J. Am. Soc. Nephrol. 6, 412–422 (2011).
Strawn, W. B., Richmond, R. S., Ann Tallant, E., Gallagher, P. E. & Ferrario, C. M. Renin-angiotensin system expression in rat bone marrow haematopoietic and stromal cells. Br. J. Haematol. 126, 120–126 (2004).
Rodgers, K. E. et al. Accelerated hematopoietic recovery with angiotensin-(1–7) after total body radiation. Int. J. Radiat. Biol. 88, 466–476 (2012).
Rodgers, K. E., Xiong, S. & diZerega, G. S. Accelerated recovery from irradiation injury by angiotensin peptides. Cancer Chemother. Pharmacol. 49, 403–411 (2002).
Wang, Y. et al. Circulating rather than cardiac angiotensin-(1–7) stimulates cardioprotection after myocardial infarction. Circ. Heart Fail. 3, 286–293 (2010).
Petty, W. J. et al. Phase I and pharmacokinetic study of angiotensin-(1–7), an endogenous antiangiogenic hormone. Clin. Cancer Res. 15, 7398–7404 (2009).
Marques, F. D. et al. An oral formulation of angiotensin-(1–7) produces cardioprotective effects in infarcted and isoproterenol-treated rats. Hypertension 57, 477–483 (2011).
de Vries, L. et al. Oral and pulmonary delivery of thioether-bridged angiotensin-(1–7). Peptides 31, 893–898 (2010).
Kluskens, L. D. et al. Angiotensin-(1–7) with thioether bridge: an angiotensin-converting enzyme-resistant, potent angiotensin-(1–7) analog. J. Pharmacol. Exp. Ther. 328, 849–854 (2009).
Durik, M. et al. The effect of the thioether-bridged, stabilized angiotensin-(1–7) analogue cyclic ang-(1–7) on cardiac remodeling and endothelial function in rats with myocardial infarction. Int. J. Hypertens. 2012, 536426 (2012).
Faria-Silva, R., Duarte, F. V. & Santos, R. A. Short-term angiotensin-(1–7) receptor MAS stimulation improves endothelial function in normotensive rats. Hypertension 46, 948–952 (2005).
Murca, T. M., Almeida, T. C., Raizada, M. K. & Ferreira, A. J. Chronic activation of endogenous angiotensin-converting enzyme 2 protects diabetic rats from cardiovascular autonomic dysfunction. Exp. Physiol. 97, 699–709 (2012).
Hernandez Prada, J. A. et al. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension 51, 1312–1317 (2008).
Meneton, P., Loffing, J. & Warnock, D. G. Sodium and potassium handling by the aldosterone-sensitive distal nephron: the pivotal role of the distal and connecting tubule. Am. J. Physiol. Renal Physiol. 287, F593–F601 (2004).
Saccomani, G., Mitchell, K. D. & Navar, L. G. Angiotensin II stimulation of Na+-H+ exchange in proximal tubule cells. Am. J. Physiol. 258, F1188–F1195 (1990).
Kim, G. H. et al. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc. Natl Acad. Sci. USA 95, 14552–14557 (1998).
Masilamani, S., Kim, G. H., Mitchell, C., Wade, J. B. & Knepper, M. A. Aldosterone-mediated regulation of ENaC α, β, and γ subunit proteins in rat kidney. J. Clin. Invest. 104, R19–R23 (1999).
Beesley, A. H., Hornby, D. & White, S. J. Regulation of distal nephron K+ channels (ROMK) mRNA expression by aldosterone in rat kidney. J. Physiol. 509 (Pt 3), 629–634 (1998).
Wald, H., Garty, H., Palmer, L. G. & Popovtzer, M. M. Differential regulation of ROMK expression in kidney cortex and medulla by aldosterone and potassium. Am. J. Physiol. 275, F239–F245 (1998).
McCormick, J. A., Yang, C. L. & Ellison, D. H. WNK kinases and renal sodium transport in health and disease: an integrated view. Hypertension 51, 588–596 (2008).
Sandberg, M. B., Riquier, A. D., Pihakaski-Maunsbach, K., McDonough, A. A. & Maunsbach, A. B. Ang II provokes acute trafficking of distal tubule Na+-Cl- cotransporter to apical membrane. Am. J. Physiol. Renal Physiol. 293, F662–F669 (2007).
van der Lubbe, N. et al. Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int. 79, 66–76 (2011).
van der Lubbe, N. et al. Aldosterone does not require angiotensin II to activate NCC through a WNK4–SPAK-dependent pathway. Pflügers Arch. 463, 853–863 (2012).
Mamenko, M., Zaika, O., Ilatovskaya, D. V., Staruschenko, A. & Pochynyuk, O. Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. J. Biol. Chem. 287, 660–671 (2012).
Sun, P., Yue, P. & Wang, W. H. Angiotensin II stimulates epithelial sodium channels in the cortical collecting duct of the rat kidney. Am. J. Physiol. Renal Physiol. 302, F679–F687 (2012).
Wei, Y., Zavilowitz, B., Satlin, L. M. & Wang, W. H. Angiotensin II inhibits the ROMK-like small conductance K channel in renal cortical collecting duct during dietary potassium restriction. J. Biol. Chem. 282, 6455–6462 (2007).
San-Cristobal, P. et al. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc. Natl Acad. Sci. USA 106, 4384–4389 (2009).
Mujais, S. K., Kauffman, S. & Katz, A. I. Angiotensin II binding sites in individual segments of the rat nephron. J. Clin. Invest. 77, 315–318 (1986).
Wang, T. & Giebisch, G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am. J. Physiol. 271, F143–F149 (1996).
Frindt, G. & Palmer, L. G. Effects of dietary K on cell-surface expression of renal ion channels and transporters. Am. J. Physiol. Renal Physiol. 299, F890–F897 (2010).
Wilson, F. H. et al. Human hypertension caused by mutations in WNK kinases. Science 293, 1107–1112 (2001).
Welling, P. A., Chang, Y. P., Delpire, E. & Wade, J. B. Multigene kinase network, kidney transport, and salt in essential hypertension. Kidney Int. 77, 1063–1069 (2010).
Castaneda-Bueno, M., Arroyo, J. P. & Gamba, G. Independent regulation of Na+ and K+ balance by the kidney. Med. Princ. Pract. 21, 101–114 (2012).
Chiga, M. et al. Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int. 74, 1403–1409 (2008).
Vallon, V., Schroth, J., Lang, F., Kuhl, D. & Uchida, S. Expression and phosphorylation of the Na+-Cl- cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am. J. Physiol. Renal Physiol. 297, F704–F712 (2009).
Yue, P. et al. Angiotensin II diminishes the effect of SGK1 on the WNK4-mediated inhibition of ROMK1 channels. Kidney Int. 79, 423–431 (2011).
Glover, M. & O'Shaughnessy, K. M. SPAK and WNK kinases: a new target for blood pressure treatment? Curr. Opin. Nephrol. Hypertens. 20, 16–22 (2011).
Arroyo, J. P., Ronzaud, C., Lagnaz, D., Staub, O. & Gamba, G. Aldosterone paradox: differential regulation of ion transport in distal nephron. Physiology (Bethesda) 26, 115–123 (2011).
Hoorn, E. J., Nelson, J. H., McCormick, J. A. & Ellison, D. H. The WNK kinase network regulating sodium, potassium, and blood pressure. J. Am. Soc. Nephrol. 22, 605–614 (2011).
O'Reilly, M. et al. Dietary electrolyte-driven responses in the renal WNK kinase pathway in vivo. J. Am. Soc. Nephrol. 17, 2402–2413 (2006).
Brater, D. C. Diuretic therapy. N. Engl. J. Med. 339, 387–395 (1998).
Vogt, L., Navis, G. & de Zeeuw, D. Renoprotection: a matter of blood pressure reduction or agent-characteristics? J. Am. Soc. Nephrol. 13 (Suppl. 3), S202–S207 (2002).
Acknowledgements
The angiotensin 1–7 research described in this review was supported by the Netherlands Heart Foundation (grant number 2010B009). E. J. Hoorn is supported by a grant from The Netherlands Organisation for Scientific Research (NWO, Veni grant 91612140).
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, contributed substantially to discussion of the content and wrote the article. A. J. M. Roks, E. J. Hoorn and A. H. J. Danser reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sevá Pessôa, B., van der Lubbe, N., Verdonk, K. et al. Key developments in renin–angiotensin–aldosterone system inhibition. Nat Rev Nephrol 9, 26–36 (2013). https://doi.org/10.1038/nrneph.2012.249
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2012.249
This article is cited by
-
The renin-angiotensin-aldosterone system (RAAS) signaling pathways and cancer: foes versus allies
Cancer Cell International (2023)
-
Crosstalk between the renin–angiotensin, complement and kallikrein–kinin systems in inflammation
Nature Reviews Immunology (2022)
-
Nitric oxide signalling in kidney regulation and cardiometabolic health
Nature Reviews Nephrology (2021)
-
The (pro)renin receptor (ATP6ap2) facilitates receptor-mediated endocytosis and lysosomal function in the renal proximal tubule
Pflügers Archiv - European Journal of Physiology (2021)
-
Comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in chemoprevention of hepatocellular carcinoma: a nationwide high-risk cohort study
BMC Cancer (2018)