Abstract
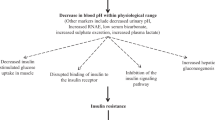
Metabolic acidosis is characterized by a primary reduction in serum bicarbonate (HCO3−) concentration, a secondary decrease in the arterial partial pressure of carbon dioxide (PaCO2) of ∼1 mmHg for every 1 mmol/l fall in serum HCO3− concentration, and a reduction in blood pH. Acute forms (lasting minutes to several days) and chronic forms (lasting weeks to years) of the disorder can occur, for which the underlying cause/s and resulting adverse effects may differ. Acute forms of metabolic acidosis most frequently result from the overproduction of organic acids such as ketoacids or lactic acid; by contrast, chronic metabolic acidosis often reflects bicarbonate wasting and/or impaired renal acidification. The calculation of the serum anion gap, calculated as [Na+] – ([HCO3−] + [Cl−]), aids diagnosis by classifying the disorders into categories of normal (hyperchloremic) anion gap or elevated anion gap. These categories can overlap, however. Adverse effects of acute metabolic acidosis primarily include decreased cardiac output, arterial dilatation with hypotension, altered oxygen delivery, decreased ATP production, predisposition to arrhythmias, and impairment of the immune response. The main adverse effects of chronic metabolic acidosis are increased muscle degradation and abnormal bone metabolism. Using base to treat acute metabolic acidosis is controversial because of a lack of definitive benefit and because of potential complications. By contrast, the administration of base for the treatment of chronic metabolic acidosis is associated with improved cellular function and few complications.
Key Points
-
Categorizing metabolic acidosis into acute and chronic varieties can be valuable for anticipating adverse effects and for determining the risks and benefits of therapy
-
A systematic approach to diagnosis of metabolic acidosis is valuable; use of the serum anion gap is an important initial tool although its limitations should be understood
-
The adverse effects of acute metabolic acidosis primarily involve the cardiovascular system, whereas the adverse effects of chronic metabolic acidosis primarily involve the musculoskeletal system
-
The treatment of acute metabolic acidosis with the administration of base has not proven beneficial in improving cardiovascular function; alternative therapies are needed
-
The treatment of chronic metabolic acidosis with the administration of base is beneficial but the goal of therapy remains unclear
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Gunnerson, K. J., Saul, M., He, S. & Kellum, J. Lactate versus non-lactate metabolic acidosis: a retrospective outcome evaluation of critically ill patients. Crit. Care Med. 10, R22–R32 (2006).
Eustace, J. A., Astor, B., Muntner, P. M., Ikizler, T. A. & Coresh, J. Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney Int. 65, 1031–1040 (2004).
Kraut, J. A. & Kurtz, I. Metabolic acidosis of CKD: diagnosis, clinical characteristics, and treatment. Am. J. Kidney Dis. 45, 978–993 (2005).
Kalantar-Zadeh, K., Mehrotra, R., Fouque, D. & Kopple, J. D. Metabolic acidosis and malnutrition-inflammation complex syndrome in chronic renal failure. Semin. Dial. 17, 455–465 (2004).
Kraut, J. A. & Kurtz, I. Controversies in the treatment of acute metabolic acidosis. NephSAP 5, 1–9 (2006).
Cohen, R. M., Feldman, G. M. & Fernandez, P. C. The balance of acid base and charge in health and disease. Kidney Int. 52, 287–293 (1997).
Rodriguez-Soriano, J. & Vallo, A. Renal tubular acidosis. Pediatr. Nephrol. 4, 268–275 (1990).
Wagner, C. A., Devuyst, O., Bourgeois, S. & Mohebbi, N. Regulated acid-base transport in the collecting duct. Pflugers Arch. 458, 137–156 (2009).
Boron, W. F. Acid base transport by the renal proximal tubule. J. Am. Soc. Nephrol. 17, 2368–2382 (2006).
Igarashi, T., Sekine, T. & Watanabe, H. Molecular basis of proximal renal tubular acidosis. J. Nephrol. 15, S135–S141 (2002).
Sly, W. S., Sato, S. & Zhu, X. L. Evaluation of carbonic anhydrase isozymes in disorders involving osteopetrosis and/or renal tubular acidosis. Clin. Biochem. 24, 311–318 (1991).
Dinour, D. et al. A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. J. Biol. Chem. 279, 52238–52246 (2004).
Wagner, C. A. et al. Renal vacuolar H+-ATPase. Physiol. Rev. 84, 1263–1314 (2004).
Gumz, M. L., Lynch, I. J., Greenlee, M. M., Cain, B. D. & Wingo, C. S. The renal H+-K+-ATPases: physiology, regulation, and structure. Am. J. Physiol. 298, F12–F21 (2010).
Karim, Z., Szutkowska, M., Vernimmen, C. & Bichara, M. Recent concepts concerning the renal handling of NH3/NH4+. J. Nephrol. 19, S27–S32 (2006).
Nagami, G. T. Ammonia production and secretion by S3 proximal tubule segments from acidotic mice: role of ANG II. Am. J. Physiol. 287, F707–F712 (2004).
Weiner, I. D. & Hamm, L. L. Molecular mechanisms of renal ammonia transport. Annu. Rev. Physiol. 69, 317–340 (2007).
Biver, S. et al. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456, 339–343 (2008).
Karet, F. E. Physiological and metabolic implications of V-ATPase isoforms in the kidney. J. Bioenerg. Biomembr. 37, 425–429 (2005).
Wagner, C. A. et al. Regulation of the expression of the Cl-/anion exchanger pendrin in mouse kidney by acid-base status. Kidney Int. 62, 2109–2117 (2002).
Petrovic, S., Wang, Z. H., Ma, L. Y. & Soleimani, M. Regulation of the apical Cl−/HCO3− exchanger pendrin in rat cortical collecting duct in metabolic acidosis. Am. J. Physiol. 284, F103–F112 (2003).
Karet, F. E. Mechanisms in hyperkalemic renal tubular acidosis. J. Am. Soc. Nephrol. 20, 251–254 (2009).
Kamel, K. S. et al. A new classification for renal defects in net acid excretion. Am. J. Kidney Dis. 29, 136–146 (1997).
Kraut, J. A. & Madias, N. E. Approach to patients with acid-base disorders. Respir. Care 46, 392–403 (2001).
Pierce, N. F. et al. The ventilatory response to acute base deficit in humans: time course during development and correction of metabolic acidosis. Ann. Intern. Med. 72, 633–640 (1970).
Wiederseiner, J. M., Muser, J., Lutz, T., Hulter, H. N. & Krapf, R. Acute metabolic acidosis: characterization and diagnosis of the disorder and the plasma potassium response. J. Am. Soc. Nephrol. 15, 1589–1596 (2004).
Madias, N. E., Schwartz, W. B. & Cohen, J. J. Maladaptive renal response to secondary hypocapnia during chronic HCl acidosis in dog. J. Clin. Invest. 60, 1393–1401 (1977).
Albert, M. S., Dell, R. B. & Winters, R. W. Quantitative displacement of acid-base equilibrium in metabolic acidosis. Ann. Intern. Med. 66, 312–322 (1967).
Asch, M. J., Dell, R. B., Williams, G. S., Cohen, M. & Winters, R. W. Time course for development of respiratory compensation in metabolic acidosis. J. Lab. Clin. Med. 73, 610–615 (1969).
Bushinsky, D. A., Coe, F. L., Katzenberg, C., Szidon, J. P. & Parks, J. H. Arterial PCO2 in chronic metabolic acidosis. Kidney Int. 22, 311–314 (1982).
Rastegar, A. Use of the ΔAG/ΔHCO3− ratio in the diagnosis of mixed acid-base disorders. J. Am. Soc. Nephrol. 18, 2429–2431 (2007).
Kraut, J. A. & Madias, N. E. Serum anion gap: its uses and limitations in clinical medicine. Clin. J. Am. Soc. Nephrol. 2, 162–174 (2007).
Emmett, M. Anion-gap interpretation: the old and the new. Nat. Clin. Pract. Nephrol. 2, 4–5 (2006).
Frohlich, J., Adam, W., Golbey, M. J. & Bernstein, M. Decreased anion gap associated with monoclonal and pseudomonoclonal gammopathy. Can. Med. Assoc. J. 114, 231–232 (1976).
Winter, S. D., Pearson, J. R., Gabow, P. A., Schultz, A. L. & Lepoff, R. B. The fall of the serum anion gap. Arch. Intern. Med. 150, 311–313 (1990).
Feldman, M., Soni, N. & Dickson, B. Influence of hypoalbuminemia or hyperalbuminemia on the serum anion gap. J. Lab. Clin. Med. 146, 317–320 (2005).
Oster, J. R., Singer, I., Contreras, G. N., Ahmad, H. I. & Vieira, C. F. Metabolic acidosis with extreme elevation of anion gap: case report and literature review. Am. J. Med. Sci. 317, 38–49 (1999).
Adrogue, H. J., Brensilver, J. & Madias, N. E. Changes in plasma anion gap during chronic metabolic acid-base disturbances. Am. J. Physiol. 235, F291–F297 (1978).
Madias, N. E., Homer, S. M., Johns, C. A. & Cohen, J. J. Hypochloremia as a consequence of anion gap metabolic acidosis. J. Lab. Clin. Med. 104, 15–23 (1984).
Kim, H. Y. et al. Clinical significance of the fractional excretion of anions in metabolic acidosis. Clin. Nephrol. 55, 448–452 (2001).
Gabow, P. A. et al. Diagnostic importance of increased serum anion gap. N. Engl. J. Med. 303, 854–858 (1980).
Uribarri, J., Oh, M. S. & Carroll, H. J. D-Lactic acidosis: a review of clinical presentation, biochemical features, and pathophysiologic mechanisms. Medicine (Baltimore) 77, 73–82 (1998).
Schelling, J. R., Howard, R. L., Winter, S. D. & Linas, S. L. Increased osmolal gap in alcoholic ketoacidosis and lactic acidosis. Ann. Intern. Med. 113, 580–582 (1990).
Kraut, J. A. & Kurtz, I. Toxic alcohol ingestions: clinical features, diagnosis, and management. Clin. J. Am. Soc. Nephrol. 3, 208–225 (2008).
Jacobsen, D. & McMartin, K. E. Methanol and ethylene glycol poisonings: mechanism of toxicity, clinical course, diagnosis and treatment. Med. Toxicol. 1, 309–334 (1986).
Winter, M. L., Ellis, M. D. & Snodgrass, W. R. Urine fluorescence using a Wood's lamp to detect the antifreeze additive sodium fluorescein: a qualitative adjunctive test in suspected ethylene glycol ingestions. Ann. Emerg. Med. 19, 663–667 (1990).
Tailor, P. et al. Recurrent high anion gap metabolic acidosis secondary to 5-oxoproline (pyroglutamic acid). Am. J. Kidney Dis. 46, E4–E10 (2005).
Batlle, D., Hizon, M., Cohen, E., Gutterman, C. & Gupta, R. The use of the urinary anion gap in the diagnosis of hyperchloremic metabolic acidosis. N. Engl. J. Med. 318, 594–599 (1988).
Richardson, R. M. A. & Halperin, M. L. The urine pH: a potentially misleading diagnostic test in patients with hyperchloremic metabolic acidosis. Am. J. Kidney Dis. 10, 140–143 (1987).
Sebastian, A., Schambelan, M., Lindenfeld, S. & Morris, R. C. Amelioration of metabolic acidosis with fludrocortisone therapy in hyporeninemic hypoaldosteronism. N. Engl. J. Med. 297, 576–583 (1977).
Goldstein, M. B., Bear, R., Richardson, R. M. A., Marsden, P. A. & Halperin, M. L. The urine anion gap a clinically useful index of ammonium excretion. Am. J. Med. Sci. 292, 198–202 (1986).
Kamel, K. S., Ethier, J. H., Richardson, R. M., Bear, R. A. & Halperin, M. L. Urine electrolytes and osmolality: when and how to use them. Am. J. Nephrol. 10, 89–102 (1990).
Kamel, K. S. & Halperin, M. L. An improved approach to the patient with metabolic acidosis: a need for four amendments. J. Nephrol. 19, S76–S85 (2006).
Dubose, T. D. Hyperkalemic hyperchloremic metabolic acidosis: pathophysiologic insights. Kidney Int. 51, 591–602 (1997).
Anderson, R. J., Potts, D. E., Gabow, P. A., Rumack, B. H. & Schrier, R. W. Unrecognized adult salicylate intoxication. Ann. Intern. Med. 85, 745–748 (1976).
Arbour, R. & Esparis, B. Osmolar gap metabolic acidosis in a 60-year-old man treated for hypoxemic respiratory failure: propylene glycol toxicity caused by escalating lorazepam infusion. Chest 118, 545–546 (2000).
Fenves, A. Z., Kirkpatrick, H. M., Patel, V. V., Sweetman, L. & Emmett, M. Increased anion gap metabolic acidosis as a result of 5-oxoproline (pyroglutamic acid): a role for acetaminophen. Clin. J. Am. Soc. Nephrol. 1, 441–447 (2006).
Chan, J. C. M., Asch, M. J., Lin, S. & Hays, D. M. Hyperalimentation with amino acid and casein hydrolysate solutions: mechanism of acidosis. JAMA 220, 1700–1705 (1972).
Chang, S. S. et al. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat. Genet. 12, 248–253 (1996).
Xie, J., Craig, L., Cobb, M. H. & Huang, C. L. Role of with-no-lysine [K] kinases in the pathogenesis of Gordon's syndrome. Pediatr. Nephrol. 21, 1231–1236 (2006).
Field, M. Intestinal ion transport and the pathophysiology of diarrhea. J. Clin. Invest. 111, 931–943 (2003).
Cieza, J., Sovero, Y., Estremadoyro, L. & Dumler, F. Electrolyte disturbances in elderly patients with severe diarrhea due to cholera. J. Am. Soc. Nephrol. 6, 1463–1467 (1995).
Igarashi, T., Sekine, T., Inatomi, J. & Seki, G. Unraveling the molecular pathogenesis of isolated proximal renal tubular acidosis. J. Am. Soc. Nephrol. 13, 2171–2177 (2002).
Laing, C. M., Toye, A. M., Capasso, G. & Unwin, R. J. Renal tubular acidosis: developments in our understanding of the molecular basis. Int. J. Biochem. Cell. Biol. 37, 1151–1161 (2005).
Pessler, F. et al. The spectrum of renal tubular acidosis in paediatric Sjogren syndrome. Rheumatology 45, 85–91 (2006).
Simpson, A. M. & Schwartz, G. J. Distal renal tubular acidosis with severe hypokalaemia probably caused by colonic H+-K+-ATPase deficiency. Arch. Dis. Child. 84, 504–507 (2001).
Hall, M. C., Koch, M. O. & McDougal, W. S. Metabolic consequences of urinary diversion through intestinal segments. Urol. Clin. North Am. 18, 725–735 (1991).
Streicher, H. Z., Gabow, P. A., Moss, A. H., Kono, D. & Kaehny, W. D. Syndromes of toluene sniffing in adults. Ann. Intern. Med. 94, 758–762 (1981).
Adrogue, H. J., Wilson, H., Boyd, A. E., Suki, W. N. & Eknoyan, G. Plasma acid-base patterns in diabetic ketoacidosis. N. Engl. J. Med. 307, 1603–1610 (1982).
Mitchell, J. H., Wildenthal, K. & Johnson, R. L. Jr. The effects of acid-base disturbances on cardiovascular and pulmonary function. Kidney Int. 1, 375–389 (1972).
Teplinsky, K., Otoole, M., Olman, M., Walley, K. R. & Wood, L. D. Effect of lactic acidosis on canine hemodynamics and left ventricular function. Am. J. Physiol. 258, H1193–H1199 (1990).
Wildenthal, K., Mierzwiak, D. S., Myers, R. W. & Mitchell, J. H. Effects of acute lactic acidosis on left ventricular performance. Am. J. Physiol. 214, 1352–1359 (1968).
Kellum, J. A., Song, M. C. & Venkataraman, R. Effects of hyperchloremic acidosis on arterial pressure and circulating inflammatory molecules in experimental sepsis. Chest 125, 243–248 (2004).
Davies, A. O. Rapid desensitization and uncoupling of human beta adrenergic receptors in an in vitro model of lactic acidosis. J. Clin. Endocrinol. Metab. 59, 398–404 (1984).
Orchard, C. H. & Cingolani, H. E. Acidosis and arrhythmias in cardiac muscle. Cardiovasc. Res. 28, 1312–1319 (1994).
Cooper, D. J., Walley, K. R., Wiggs, B. R. & Russell, J. A. Bicarbonate does not improve hemodynamics in critically ill patients who have lactic acidosis. Ann. Intern. Med. 112, 492–498 (1990).
Mathieu, D., Neviere, R., Billard, V., Fleyfel, M. & Wattel, F. Effects of bicarbonate therapy on hemodynamics and tissue oxygenation in patients with lactic acidosis: a prospective, controlled clinical study. Crit. Care Med. 19, 1352–1356 (1991).
Khazel, A., McLaughlin, J. S., Suddhimonadala, C., Atar, S. & Cowley, R. A. The effects of acidosis and alkalosis on cardiac output and peripheral resistance in humans. Am. Surg. 35, 600–605 (1969).
Seifter, J. Acid base disturbances and the central nervous system. Nephrol. Rounds 3, 1–6 (2005).
Bellingham, A. J., Detter, J. C. & Lenfant, C. Regulatory mechanisms of hemoglobin oxygen affinity in acidosis and alkalosis. J. Clin. Invest. 50, 700–706 (1971).
Kellum, J. A., Song, M. C. & Li, J. Y. Science review: extracellular acidosis and the immune response: clinical and physiologic implications. Crit. Care 8, 331–336 (2004).
Lardner, A. The effects of extracellular pH on immune function. J. Leukoc. Biol. 69, 522–530 (2001).
Cuthbert, C. & Alberti, K. G. Acidemia and insulin resistance in the diabetic ketoacidotic rat. Metabolism 27, 1903–1916 (1978).
Halperin, F. A., Cheema-Dhadli, S., Chen, C. B. & Halperin, M. I. Alkali therapy extends the period of survival during hypoxia: studies in rats. Am. J. Physiol. 271, R381–R387 (1996).
Kubasiak, L. A., Hernandez, O. M., Bishopric, N. H. & Webster, K. A. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc. Natl Acad. Sci. USA 99, 12825–12830 (2002).
Kovesdy, C. P., Anderson, J. E. & Kalantar-Zadeh, K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol. Dial. Transplant. 24, 1232–1237 (2009).
Kraut, J. A. Disturbances of acid-base balance and bone disease in end-stage renal disease. Semin. Dial. 13, 261–265 (2000).
Lemann, J., Bushinsky, D. A. & Hamm, L. L. Bone buffering of acid and base in humans. Am. J. Physiol. 285, F811–F832 (2003).
Mitch, W. E. Proteolytic mechanisms, not malnutrition, cause loss of muscle mass in kidney failure. J. Ren. Nutr. 16, 208–211 (2006).
McSherry, E. & Morris, R. C. Attainment and maintenance of normal stature with alkali therapy in infants and children with classic renal tubular acidosis. J. Clin. Invest. 61, 509–527 (1978).
Mak, R. H. Insulin and its role in chronic kidney disease. Pediatr. Nephrol. 23, 355–362 (2008).
Shah, S. N., Abramowitz, M., Hostetter, T. H. & Melamed, M. H. S. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am. J. Kidney Dis. 54, 270–277 (2009).
Sonikian, M. et al. Potential effect of metabolic acidosis on beta 2-microglobulin generation: in vivo and in vitro studies. J. Am. Soc. Nephrol. 7, 350–356 (1996).
Wiederkehr, M. R., Kalogiros, J. & Krapf, R. Correction of metabolic acidosis improves thyroid and growth hormone axes in haemodialysis patients. Nephrol. Dial. Transplant. 19, 1190–1197 (2004).
Mitch, W. E. Metabolic and clinical consequences of metabolic acidosis. J. Nephrol. 19, S70–S75 (2006).
Sebastian, A., Harris, S. T., Ottaway, J. H., Todd, K. M. & Morris, R. C. Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. N. Engl. J. Med. 330, 1776–1781 (1994).
Frassetto, L., Morris, R. C. & Sebastian, A. Potassium bicarbonate reduces urinary nitrogen excretion in postmenopausal women. J. Clin. Endocrinol. Metab. 82, 254–259 (1997).
Kraut, J. A. & Kurtz, I. Use of base in the treatment of severe acidemic states. Am. J. Kidney Dis. 38, 703–727 (2001).
Forsythe, S. & Schmidt, G. A. Sodium bicarbonate for the treatment of lactic acidosis. Chest 117, 260–267 (2000).
Glaser, N. et al. Risk factors for cerebral edema in children with diabetic ketoacidosis. N. Engl. J. Med. 344, 264–269 (2001).
Kraut, J. A. & Kurtz, I. Use of base in the treatment of acute severe organic acidosis by nephrologists and critical care physicians: results of an online survey. Clin. Exp. Nephrol. 10, 111–117 (2006).
Wu, D. M. et al. Na+/H+ exchange inhibition delays the onset of hypovolemic circulatory shock in pigs. Shock 29, 519–525 (2008).
Nahas, G. G., Sutin, K. M. & Fermon, C. Guidelines for the treatment of acidaemia with THAM. Drugs 55, 191–194 (1998).
Hoste, E. A. et al. Sodium bicarbonate versus THAM in ICU patients with mild metabolic acidosis. J. Nephrol. 18, 303–307 (2005).
Weber, T. et al. Tromethamine buffer modifies the depressant effect of permissive hypercapnia on myocardial contractility in patient with acute respiratory distress syndrome. Am. J. Resp. Crit. Care Med. 162, 1361–1365 (2000).
Klepper, I. D., Kucera, R. F., Kindig, N. B., Sherrill, D. L. & Filley, G. F. A comparative study of bicarbonate and Carbicarb in the treatment of metabolic acidosis induced by hemorrhagic shock. J. Crit. Care 3, 256–261 (1988).
Zhou, F. Q. Pyruvate in the correction of intracellular acidosis: a metabolic basis as a novel superior buffer. Am. J. Nephrol. 25, 55–63 (2005).
Hilton, P. J., Taylor, L. G., Forni, L. G. & Treacher, D. F. Bicarbonate-based haemofiltration in the management of acute renal failure with lactic acidosis. QJM 91, 279–283 (1998).
Wu, D. M., Bassuk, J., Arias, J., Doods, H. & Adams, J. A. Cardiovascular effects of Na+/H+ exchanger inhibition with BIIB513 following hypovolemic circulatory shock. Shock 23, 269–274 (2005).
Sikes, P. J., Zhao, P., Maass, D. L., White, J. & Horton, J. W. Sodium/hydrogen exchange activity in sepsis and in sepsis complicated by previous injury: 31P and 23Na NMR study. Crit. Care Med. 33, 605–615 (2005).
Benveniste, M. & Dingledine, R. Limiting stroke-induced damage by targeting an acid channel. N. Engl. J. Med. 352, 85–86 (2005).
Xiong, Z. G., Chu, X. P. & Simon, R. P. Acid sensing ion channels: novel therapeutic targets for ischemic brain injury. Front. Biosci. 12, 1376–1386 (2007).
Sabatini, S. & Kurtzman, N. A. Bicarbonate therapy in severe metabolic acidosis. J. Am. Soc. Nephrol. 20, 692–695 (2009).
Garella, S., Dana, C. L. & Chazan, J. A. Severity of metabolic acidosis as a determinant of bicarbonate requirements. N. Engl. J. Med. 289, 121–126 (1973).
Fernandez, P. C., Cohen, R. M. & Feldman, G. M. The concept of bicarbonate distribution space: the crucial role of body buffers. Kidney Int. 36, 747–752 (1989).
Kallet, R. H., Jasmer, R. M., Luce, J. M., Lin, L. H. & Marks, J. D. The treatment of acidosis in acute lung injury with tris-hydroxymethyl aminomethane (THAM). Am. J. Resp. Crit. Care Med. 161, 1149–1153 (2000).
Adrogue, H. J., Rashad, M. N., Gorin, A. B., Yacoub, J. & Madias, N. E. Assessing acid-base status in circulatory failure. Differences between arterial and central venous blood. N. Engl. J. Med. 320, 1312–1316 (1989).
Roderick, P., Willis, N. S., Blakeley, S., Jones, C. & Tomson, C. Correction of chronic metabolic acidosis for chronic kidney disease patients. Cochrane Database of Systematic Reviews, Issue 1. Art. No.: CD001890. doi:10.1002/14651858.CD001890.pub3 (2007).
Ballmer, P. E. et al. Chronic metabolic acidosis decreases albumin synthesis and induces negative nitrogen balance in humans. J. Clin. Invest. 95, 39–45 (1995).
de Brito-Ashurst, I., Varagunam, M., Raftery, M. J. & Yaqoob, M. M. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J. Am. Soc. Nephrol. 20, 2075–2084 (2009).
Husted, F. C. & Nolph, K. D. NaHCO3− and NaCl tolerance in chronic renal failure II. Clin. Nephrol. 1, 21–27 (1977).
Szylman, P., Better, O. S., Chaimowitz, C. & Rosler, A. Role of hyperkalemia in the metabolic acidosis of isolated hypoaldosteronism. N. Engl. J. Med. 294, 361–365 (1976).
Harris, D. C. H., Yuill, E. & Chesher, D. W. Correcting acidosis in hemodialysis: effect on phosphate clearance and calcification risk. J. Am. Soc. Nephrol. 6, 1607–1612 (1995).
Kopple, J. D., Kalantar-Zadeh, K. & Mehrotra, R. Risks of chronic metabolic acidosis in patients with chronic kidney disease. Kidney Int. 67, S21–S27 (2005).
Acknowledgements
J. A. Kraut's work is supported in part by research funds from the Veterans Administration.
C. P. Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the MedscapeCME-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kraut, J., Madias, N. Metabolic acidosis: pathophysiology, diagnosis and management. Nat Rev Nephrol 6, 274–285 (2010). https://doi.org/10.1038/nrneph.2010.33
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2010.33
This article is cited by
-
The synergism of cytosolic acidosis and reduced NAD+/NADH ratio is responsible for lactic acidosis-induced vascular smooth muscle cell impairment in sepsis
Journal of Biomedical Science (2024)
-
The impact of diabetes, anemia, and renal function in the relationship between osteoporosis and fasting blood glucose among Taiwanese women: a cross-sectional study
BMC Women's Health (2024)
-
A severe pediatric life-threatening metabolic ketoacidosis
Journal of Diabetes & Metabolic Disorders (2024)
-
Clinical efficacy of sodium bicarbonate in treating pediatric metabolic acidosis with varying level of acid–base balance parameters: a real-world study
BMC Medicine (2023)
-
Transient loss of consciousness immediately after total pancreatectomy for pancreatic metastases from renal cell carcinoma: a case report
Surgical Case Reports (2023)