Abstract
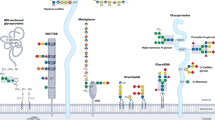
The glomerulus consists of capillary tufts, a mesangial cell component and the Bowman capsule. The glomerular filtration barrier is composed of glomerular endothelial cells, a basement membrane, and podocytes. Particular components of the slit diaphragm and the glomerular basement membrane strictly orchestrate the integrity of the glomerular filtration barrier. The basement membrane is made of a highly crosslinked macromolecular meshwork of type IV collagen, proteoglycans, and laminin. Genetic forms of glomerular disease are predominantly caused by genetic defects in these molecular structures or in factors that regulate the glomerular filtration barrier. In addition, abnormal IgA1 glycosylation can increase susceptibility to IgA nephropathy. Dysregulation of the complement system or of platelet activation can lead to the development of endocapillary lesions, which manifest as thrombotic microangiopathy. Glomerular dysfunction is also encountered in several genetic metabolic and mitochondrial disorders. Discoveries of mutations in a range of genes have provided new insights into the mechanisms of glomerular disease. In this Review, we summarize recent progress in the genetics and therapeutics of a number of glomerular diseases.
Key Points
-
Genetic defects in glomerular endocapillaries, the glomerular basement membrane, and podocytes all contribute to the pathogenesis of congenital glomerulopathies
-
Systemic genetic diseases, for example those caused by defects in metabolism, mitochondrial energy depletion, and immune diseases, can also result in glomerulopathy
-
Novel genome-wide association and systems biology studies have improved our understanding of monogenic glomerulopathies in complex kidney diseases
-
Transgenic mice and gene therapy will expand our understanding of the molecular structure of the glomerular filtration barrier, and will probably reveal novel therapeutic strategies for the treatment of genetic glomerular diseases
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Yamada, E. The fine structure of the renal glomerulus of the mouse. J. Histochem. Cytochem. 3, 309 (1955).
Ohse, T. et al. A new function for parietal epithelial cells: a second glomerular barrier. Am. J. Physiol. Renal Physiol. 297, F1566–F1574 (2009).
Ohse, T. et al. The enigmatic parietal epithelial cell is finally getting noticed: a review. Kidney Int. 76, 1225–1238 (2009).
Schlondorff, D. & Banas, B. The mesangial cell revisited: no cell is an island. J. Am. Soc. Nephrol. 20, 1179–1187 (2009).
Ciechanowicz, A., Brodkiewicz, A., Binczak-Kuleta, A., Parczewski, M. & Czekalski, S. “Treasure your exceptions”: recent advances in molecular genetics of glomerular disease. J. Appl. Genet. 49, 93–99 (2008).
Lavin, P. J., Gbadegesin, R., Damodaran, T. V. & Winn, M. P. Therapeutic targets in focal and segmental glomerulosclerosis. Curr. Opin. Nephrol. Hypertens. 17, 386–392 (2008).
Patrakka, J. & Tryggvason, K. New insights into the role of podocytes in proteinuria. Nat. Rev. Nephrol. 5, 463–468 (2009).
Tryggvason, K., Patrakka, J. & Wartiovaara, J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N. Engl. J. Med. 354, 1387–1401 (2006).
Kestila, M. et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol. Cell 1, 575–582 (1998).
Ahvenainen, E. K., Hallman, N. & Hjelt, L. Nephrotic syndrome in newborn and young infants. Ann. Paediatr. Fenn. 2, 227–241 (1956).
Beltcheva, O., Martin, P., Lenkkeri, U. & Tryggvason, K. Mutation spectrum in the nephrin gene (NPHS1) in congenital nephrotic syndrome. Hum. Mutat. 17, 368–373 (2001).
Ruotsalainen, V. et al. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc. Natl Acad. Sci. USA 96, 7962–7967 (1999).
Olsen, A. S., Georgescu, A., Johnson, S. & Carrano, A. V. Assembly of a 1-Mb restriction-mapped cosmid contig spanning the candidate region for Finnish congenital nephrosis (NPHS1) in 19q13.1. Genomics 34, 223–225 (1996).
Lenkkeri, U. et al. Structure of the gene for congenital nephrotic syndrome of the finnish type (NPHS1) and characterization of mutations. Am. J. Hum. Genet. 64, 51–61 (1999).
Holzman, L. B. et al. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 56, 1481–1491 (1999).
Lahdenpera, J. et al. Clustering-induced tyrosine phosphorylation of nephrin by Src family kinases. Kidney Int. 64, 404–413 (2003).
Verma, R. et al. Fyn binds to and phosphorylates the kidney slit diaphragm component nephrin. J. Biol. Chem. 278, 20716–20723 (2003).
Li, H., Lemay, S., Aoudjit, L., Kawachi, H. & Takano, T. SRC-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J. Am. Soc. Nephrol. 15, 3006–3015 (2004).
Koziell, A. et al. Genotype/phenotype correlations of NPHS1 and NPHS2 mutations in nephrotic syndrome advocate a functional inter-relationship in glomerular filtration. Hum. Mol. Genet. 11, 379–388 (2002).
Boute, N. et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 24, 349–354 (2000).
Roselli, S. et al. Podocin localizes in the kidney to the slit diaphragm area. Am. J. Pathol. 160, 131–139 (2002).
Roselli, S. et al. Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol. Cell Biol. 24, 550–560 (2004).
Shih, N. Y. et al. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286, 312–315 (1999).
Fukasawa, H., Bornheimer, S., Kudlicka, K. & Farquhar, M. G. Slit diaphragms contain tight junction proteins. J. Am. Soc. Nephrol. 20, 1491–1503 (2009).
Hofmann, T. et al. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397, 259–263 (1999).
Reiser, J. et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 37, 739–744 (2005).
Fuchshuber, A. et al. Mapping a gene (SRN1) to chromosome 1q25-q31 in idiopathic nephrotic syndrome confirms a distinct entity of autosomal recessive nephrosis. Hum. Mol. Genet. 4, 2155–2158 (1995).
Hinkes, B. G. et al. Nephrotic syndrome in the first year of life: two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2). Pediatrics 119, e907–e919 (2007).
Nagase, T., Kikuno, R., Ishikawa, K., Hirosawa, M. & Ohara, O. Prediction of the coding sequences of unidentified human genes. XVII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 7, 143–150 (2000).
Hinkes, B. et al. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat. Genet. 38, 1397–1405 (2006).
Zhou, W. & Hildebrandt, F. Molecular cloning and expression of phospholipase C epsilon 1 in zebrafish. Gene Expr. Patterns 9, 282–288 (2009).
Shih, N. Y. et al. CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am. J. Pathol. 159, 2303–2308 (2001).
Dustin, M. L. et al. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell 94, 667–677 (1998).
Kim, J. M. et al. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science 300, 1298–1300 (2003).
Gigante, M. et al. CD2AP mutations are associated with sporadic nephrotic syndrome and focal segmental glomerulosclerosis (FSGS). Nephrol. Dial. Transplant. 24, 1858–1864 (2009).
Löwik, M. et al. Bigenic heterozygosity and the development of steroid-resistant focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 23, 3146–3151 (2008).
Löwik, M. M. et al. Focal segmental glomerulosclerosis in a patient homozygous for a CD2AP mutation. Kidney Int. 72, 1198–1203 (2007).
Honda, K. et al. Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J. Cell. Biol. 140, 1383–1393 (1998).
Kaplan, J. M. et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat. Genet. 24, 251–256 (2000).
Weins, A. et al. Disease-associated mutant alpha-actinin-4 reveals a mechanism for regulating its F-actin-binding affinity. Proc. Natl Acad. Sci. USA 104, 16080–16085 (2007).
Ihalmo, P. et al. Association analysis of podocyte slit diaphragm genes as candidates for diabetic nephropathy. Diabetologia 51, 86–90 (2008).
Woudenberg-Vrenken, T. E., Bindels, R. J. & Hoenderop, J. G. The role of transient receptor potential channels in kidney disease. Nat. Rev. Nephrol. 5, 441–449 (2009).
Winn, M. P. et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801–1804 (2005).
Dietrich, A. et al. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol. Cell Biol. 25, 6980–6989 (2005).
Moller, C. C. et al. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J. Am. Soc. Nephrol. 18, 29–36 (2007).
Spassova, M. A., Hewavitharana, T., Xu, W., Soboloff, J. & Gill, D. L. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc. Natl Acad. Sci. USA 103, 16586–16591 (2006).
Zenker, M., Machuca, E. & Antignac, C. Genetics of nephrotic syndrome: new insights into molecules acting at the glomerular filtration barrier. J. Mol. Med. 87, 849–857 (2009).
Epstein, C. J. et al. Hereditary macrothrombocytopathia, nephritis and deafness. Am. J. Med. 52, 299–310 (1972).
Peterson, L. C., Rao, K. V., Crosson, J. T. & White, J. G. Fechtner syndrome—a variant of Alport's syndrome with leukocyte inclusions and macrothrombocytopenia. Blood 65, 397–406 (1985).
Saez, C. G., Myers, J. C., Shows, T. B. & Leinwand, L. A. Human nonmuscle myosin heavy chain mRNA: generation of diversity through alternative polyadenylylation. Proc. Natl Acad. Sci. USA 87, 1164–1168 (1990).
Gershoni-Baruch, R., Baruch, Y., Viener, A. & Lichtig, C. Fechtner syndrome: clinical and genetic aspects. Am. J. Med. Genet. 31, 357–367 (1988).
Kao, W. H. et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat. Genet. 40, 1185–1192 (2008).
Kopp, J. B. et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat. Genet. 40, 1175–1184 (2008).
Matsushita, T. et al. Targeted disruption of mouse ortholog of the human MYH9 responsible for macrothrombocytopenia with different organ involvement: hematological, nephrological, and otological studies of heterozygous KO mice. Biochem. Biophys. Res. Commun. 325, 1163–1171 (2004).
Haber, D. A. & Buckler, A. J. WT1: a novel tumor suppressor gene inactivated in Wilms' tumor. New Biol. 4, 97–106 (1992).
Haber, D. A. et al. An internal deletion within an 11p13 zinc finger gene contributes to the development of Wilms' tumor. Cell 61, 1257–1269 (1990).
Call, K. M. et al. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell 60, 509–520 (1990).
Morrison, A. A., Viney, R. L., Saleem, M. A. & Ladomery, M. R. New insights into the function of the Wilms tumor suppressor gene WT1 in podocytes. Am. J. Physiol. Renal Physiol. 295, F12–F17 (2008).
Pelletier, J. et al. Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell 67, 437–447 (1991).
Coleman, M. A., Eisen, J. A. & Mohrenweiser, H. W. Cloning and characterization of HARP/SMARCAL1: a prokaryotic HepA-related SNF2 helicase protein from human and mouse. Genomics 65, 274–282 (2000).
Gubler, M. C. Inherited diseases of the glomerular basement membrane. Nat. Clin. Pract. Nephrol. 4, 24–37 (2008).
Goldberg, S., Harvey, S. J., Cunningham, J., Tryggvason, K. & Miner, J. H. Glomerular filtration is normal in the absence of both agrin and perlecanheparan sulfate from the glomerular basement membrane. Nephrol. Dial. Transplant. 24, 2044–2051 (2009).
Beirowski, B., Weber, M. & Gross, O. Chronic renal failure and shortened lifespan in COL4A3± mice: an animal model for thin basement membrane nephropathy. J. Am. Soc. Nephrol. 17, 1986–1994 (2006).
Hudson, B. G., Tryggvason, K., Sundaramoorthy, M. & Neilson, E. G. Alport's syndrome, Goodpasture's syndrome, and type IV collagen. N. Engl. J. Med. 348, 2543–2556 (2003).
Timpl, R. & Brown, J. C. The laminins. Matrix Biol. 14, 275–281 (1994).
Alport, A. C. Hereditary familial congenital haemorrhagic nephritis. BMJ I, 504–506 (1927).
Rayat, C. S. et al. Glomerular morphometry in biopsy evaluation of minimal change disease, membranous glomerulonephritis, thin basement membrane disease and Alport's syndrome. Anal. Quant. Cytol. Histol. 29, 173–182 (2007).
Barker, D. F. et al. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science 248, 1224–1227 (1990).
Hostikka, S. L. et al. Identification of a distinct type IV collagen alpha chain with restricted kidney distribution and assignment of its gene to the locus of X chromosome-linked Alport syndrome. Proc. Natl Acad. Sci. USA, 87, 1606–1610 (1990).
Myers, J. C. et al. Molecular cloning of alpha 5(IV) collagen and assignment of the gene to the region of the X chromosome containing the Alport syndrome locus. Am. J. Hum. Genet. 46, 1024–1033 (1990).
Heidet, L. & Gubler, M. C. The renal lesions of Alport syndrome. J. Am. Soc. Nephrol. 20, 1210–1215 (2009).
Perkoff, G. T., Stephens, F. E., Dolowitz, D. A. & Tyler, F. H. A clinical study of hereditary interstitial pyelonephritis. AMA Arch. Intern. Med. 88, 191–200 (1951).
Kashtan, C. E. Alport syndrome. An inherited disorder of renal, ocular, and cochlear basement membranes. Medicine (Baltimore) 78, 338–360 (1999).
Zhou, J. et al. Deletion of the paired alpha 5(IV) and alpha 6(IV) collagen genes in inherited smooth muscle tumors. Science 261, 1167–1169 (1993).
Mochizuki, T. et al. Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat. Genet. 8, 77–81 (1994).
Lemmink, H. H. et al. Mutations in the type IV collagen alpha 3 (COL4A3) gene in autosomal recessive Alport syndrome. Hum. Mol. Genet. 3, 1269–1273 (1994).
van der Loop, F. T. et al. Autosomal dominant Alport syndrome caused by a COL4A3 splice site mutation. Kidney Int. 58, 1870–1875 (2000).
Pescucci, C. et al. Autosomal-dominant Alport syndrome: natural history of a disease due to COL4A3 or COL4A4 gene. Kidney Int. 65, 1598–1603 (2004).
Tryggvason, K. & Patrakka, J. Thin basement membrane nephropathy. J. Am. Soc. Nephrol. 17, 813–822 (2006).
Mariyama, M., Zheng, K., Yang-Feng, T. L. & Reeders, S. T. Colocalization of the genes for the alpha 3(IV) and alpha 4(IV) chains of type IV collagen to chromosome 2 bands q35-q37. Genomics 13, 809–813 (1992).
Haas, M. Alport syndrome and thin glomerular basement membrane nephropathy: a practical approach to diagnosis. Arch. Pathol. Lab. Med. 133, 224–232 (2009).
Zenker, M. et al. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum. Mol. Genet. 13, 2625–2632 (2004).
Zenker, M. et al. Congenital nephrosis, mesangial sclerosis, and distinct eye abnormalities with microcoria: an autosomal recessive syndrome. Am. J. Med. Genet. A 130A, 138–145 (2004).
Hasselbacher, K. et al. Recessive missense mutations in LAMB2 expand the clinical spectrum of LAMB2-associated disorders. Kidney Int. 70, 1008–1012 (2006).
Noakes, P. G. et al. The renal glomerulus of mice lacking s-laminin/laminin beta 2: nephrosis despite molecular compensation by laminin beta 1. Nat. Genet. 10, 400–406 (1995).
Bongers, E. M. et al. Genotype-phenotype studies in nail-patella syndrome show that LMX1B mutation location is involved in the risk of developing nephropathy. Eur. J. Hum. Genet. 13, 935–946 (2005).
Miner, J. H. et al. Transcriptional induction of slit diaphragm genes by Lmx1b is required in podocyte differentiation. J. Clin. Invest. 109, 1065–1072 (2002).
Suleiman, H. et al. The podocyte-specific inactivation of Lmx1b, Ldb1 and E2a yields new insight into a transcriptional network in podocytes. Dev. Biol. 304, 701–712 (2007).
Chen, H. et al. Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat. Genet. 19, 51–55 (1998).
Chen, H. et al. Multiple calvarial defects in lmx1b mutant mice. Dev. Genet. 22, 314–320 (1998).
Heidet, L. et al. In vivo expression of putative LMX1B targets in nail-patella syndrome kidneys. Am. J. Pathol. 163, 145–155 (2003).
Barratt, J. & Eitner, F. Glomerular disease: sugars and immune complex formation in IgA nephropathy. Nat. Rev. Nephrol. 5, 612–614 (2009).
Suzuki, H. et al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J. Clin. Invest. 119, 1668–1677 (2009).
Gharavi, A. G. et al. IgA nephropathy, the most common cause of glomerulonephritis, is linked to 6q22–23 Nat. Genet. 26, 354–357 (2000).
Bisceglia, L. et al. Genetic heterogeneity in Italian families with IgA nephropathy: suggestive linkage for two novel IgA nephropathy loci. Am. J. Hum. Genet. 79, 1130–1134 (2006).
Hiki, Y. et al. O-linked oligosaccharide on IgA1 hinge region in IgA nephropathy. Fundamental study for precise structure and possible role. Contrib. Nephrol. 111, 73–84 (1995).
Hiki, Y. et al. Association of asialo-galactosyl beta 1-3N-acetylgalactosamine on the hinge with a conformational instability of Jacalin-reactive immunoglobulin A1 in immunoglobulin A nephropathy. J. Am. Soc. Nephrol. 7, 955–960 (1996).
Furlan, M. et al. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N. Engl. J. Med. 339, 1578–1584 (1998).
Levy, G. G. et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature 413, 488–494 (2001).
Fujioka, M. et al. ADAMTS13 gene deletion aggravates ischemic brain damage: a possible neuroprotective role of ADAMTS13 by ameliorating post-ischemic hypoperfusion. Blood 115, 1650–1653 (2010).
Zhao, B. Q. et al. von Willebrand factor-cleaving protease ADAMTS13 reduces ischemic brain injury in experimental stroke. Blood 114, 3329–3334 (2009).
Warwicker, P. et al. Genetic studies into inherited and sporadic hemolytic uremic syndrome. Kidney Int. 53, 836–844 (1998).
Jozsi, M. et al. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood 111, 1512–1514 (2008).
Zipfel, P. F. et al. Deletion of complement factor H-related genes CFHR1 and CFHR3 is associated with atypical hemolytic uremic syndrome. PLoS Genet. 3, e41 (2007).
Noris, M. et al. Familial haemolytic uraemic syndrome and an MCP mutation. Lancet 362, 1542–1547 (2003).
Caprioli, J. et al. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood 108, 1267–1279 (2006).
Carreras, L. et al. Familial hypocomplementemic hemolytic uremic syndrome with HLA-A3, B7 haplotype. JAMA 245, 602–604 (1981).
Goicoechea de Jorge, E. et al. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc. Natl Acad. Sci. USA 104, 240–245 (2007).
Fremeaux-Bacchi, V. et al. Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood 112, 4948–4952 (2008).
Delvaeye, M. et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 361, 345–357 (2009).
Noris, M. & Remuzzi, G. Atypical hemolytic-uremic syndrome. N. Engl. J. Med. 361, 1676–1687 (2009).
Licht, C. & Fremeaux-Bacchi, V. Hereditary and acquired complement dysregulation in membranoproliferative glomerulonephritis. Thromb. Haemost. 101, 271–278 (2009).
Maeda, S. et al. Genetic variations associated with diabetic nephropathy and type II diabetes in a Japanese population. Kidney Int. Suppl. S43–S48 (2007).
Ng, D. P. et al. Genetic variation at the SLC12A3 locus is unlikely to explain risk for advanced diabetic nephropathy in Caucasians with type 2 diabetes. Nephrol. Dial. Transplant. 23, 2260–2264 (2008).
Shimazaki, A. et al. ELMO1 increases expression of extracellular matrix proteins and inhibits cell adhesion to ECMs. Kidney Int. 70, 1769–1776 (2006).
Pezzolesi, M. G. et al. Confirmation of genetic associations at ELMO1 in the GoKinD collection supports its role as a susceptibility gene in diabetic nephropathy. Diabetes 58, 2698–2702 (2009).
Pezzolesi, M. G. et al. Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes 58, 1403–1410 (2009).
Ni, X. et al. Molecular cloning and characterization of the protein 4.1O gene, a novel member of the protein 4.1 family with focal expression in ovary. J. Hum. Genet. 48, 101–106 (2003).
Ramez, M. et al. Distinct distribution of specific members of protein 4.1 gene family in the mouse nephron. Kidney Int. 63, 1321–1337 (2003).
Antonellis, A. & Green, E. D. The role of aminoacyl-tRNA synthetases in genetic diseases. Annu. Rev. Genomics Hum. Genet. 9, 87–107 (2008).
Saito, T. et al. Lipoprotein glomerulopathy: glomerular lipoprotein thrombi in a patient with hyperlipoproteinemia. Am. J. Kidney Dis. 13, 148–153 (1989).
Saito, T. [Lipoprotein glomerulopathy]. Nippon Jinzo Gakkai Shi (Suppl. 50th Ann.) 112–116 (2007).
Ishimura, A. et al. Lipoprotein glomerulopathy induced by ApoE-Sendai is different from glomerular lesions in aged apoE-deficient mice. Clin. Exp. Nephrol. 13, 430–437 (2009).
Saito, T., Matsunaga, A. & Oikawa, S. Impact of lipoprotein glomerulopathy on the relationship between lipids and renal diseases. Am. J. Kidney Dis. 47, 199–211 (2006).
Opitz, J. M. et al. the genetics of angiokeratoma corporis diffusum (Fabry's disease) and its linkage relations with the Xg locus. Am. J. Hum. Genet. 17, 325–342 (1965).
Martins, A. M. et al. Guidelines to diagnosis and monitoring of Fabry disease and review of treatment experiences. J. Pediatr. 155, S19–S31 (2009).
Gotoda, T. et al. Differential phenotypic expression by three mutant alleles in familial lecithin:cholesterol acyltransferase deficiency. Lancet 338, 778–781 (1991).
Jahanzad, I., Amoueian, S. & Attaranzadeh, A. Familial lecithin-cholesterol acyltransferase deficiency. Arch. Iran Med. 12, 179–181 (2009).
Lei, K. J., Shelly, L. L., Pan, C. J., Sidbury, J. B. & Chou, J. Y. Mutations in the glucose-6-phosphatase gene that cause glycogen storage disease type 1a. Science 262, 580–583 (1993).
Talente, G. M. et al. Glycogen storage disease in adults. Ann. Intern. Med. 120, 218–226 (1994).
Yiu, W. H. et al. Angiotensin mediates renal fibrosis in the nephropathy of glycogen storage disease type Ia. Kidney Int. 73, 716–723 (2008).
Rahman, S., Hargreaves, I., Clayton, P. & Heales, S. Neonatal presentation of coenzyme Q10 deficiency. J. Pediatr. 139, 456–458 (2001).
Salviati, L. et al. Infantile encephalomyopathy and nephropathy with CoQ10 deficiency: a CoQ10-responsive condition. Neurology 65, 606–608 (2005).
Diomedi-Camassei, F. et al. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J. Am. Soc. Nephrol. 18, 2773–2780 (2007).
Lopez, L. C. et al. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am. J. Hum. Genet. 79, 1125–1129 (2006).
Peng, M. et al. Primary coenzyme Q deficiency in Pdss2 mutant mice causes isolated renal disease. PLoS Genet. 4, e1000061 (2008).
Saiki, R. et al. Coenzyme Q10 supplementation rescues renal disease in Pdss2kd/kd mice with mutations in prenyl diphosphate synthase subunit 2. Am. J. Physiol. Renal Physiol. 295, F1535–F1544 (2008).
Yorifuji, T. et al. Nephropathy and growth hormone deficiency in a patient with mitochondrial tRNA(Leu(UUR)) mutation. J. Med. Genet. 33, 621–622 (1996).
Lowik, M. M., Hol, F. A., Steenbergen, E. J., Wetzels, J. F. & van den Heuvel, L. P. Mitochondrial tRNALeu(UUR) mutation in a patient with steroid-resistant nephrotic syndrome and focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 20, 336–341 (2005).
Kurogouchi, F. et al. A case of mitochondrial cytopathy with a typical point mutation for MELAS, presenting with severe focal-segmental glomerulosclerosis as main clinical manifestation. Am. J. Nephrol. 18, 551–556 (1998).
Jansen, J. J. et al. Mutation in mitochondrial tRNA(Leu(UUR)) gene associated with progressive kidney disease. J. Am. Soc. Nephrol. 8, 1118–1124 (1997).
Quintero-Del-Rio, A. I., Kelly, J. A., Kilpatrick, J., James, J. A. & Harley, J. B. The genetics of systemic lupus erythematosus stratified by renal disease: linkage at 10q22.3 (SLEN1), 2q34–35 (SLEN2), and 11p15.6 (SLEN3). Genes Immun. 3 (Suppl. 1), S57–S62 (2002).
Morel, L. Genetics of human lupus nephritis. Semin. Nephrol. 27, 2–11 (2007).
Borgmann, S. & Haubitz, M. Genetic impact of pathogenesis and prognosis of ANCA-associated vasculitides. Clin. Exp. Rheumatol. 22, S79–S86 (2004).
Jagiello, P. et al. New genomic region for Wegener's granulomatosis as revealed by an extended association screen with 202 apoptosis-related genes. Hum. Genet. 114, 468–477 (2004).
Brown, E. J. et al. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat. Genet. 42, 72–76 (2010).
Kambham, N. et al. Congenital focal segmental glomerulosclerosis associated with beta4 integrin mutation and epidermolysis bullosa. Am. J. Kidney Dis. 36, 190–196 (2000).
Agarwal, A. K. et al. Focal segmental glomerulosclerosis in patients with mandibuloacral dysplasia owing to ZMPSTE24 deficiency. J. Investig. Med. 54, 208–213 (2006).
Berkovic, S. F. et al. Array-based gene discovery with three unrelated subjects shows SCARB2/LIMP-2 deficiency causes myoclonus epilepsy and glomerulosclerosis. Am. J. Hum. Genet. 82, 673–684 (2008).
Balreira, A. et al. A nonsense mutation in the LIMP-2 gene associated with progressive myoclonic epilepsy and nephrotic syndrome. Hum. Mol. Genet. 17, 2238–2243 (2008).
Copelovitch, L., Guttenberg, M., Pollak, M. R. & Kaplan, B. S. Reninangiotensin axis blockade reduces proteinuria in presymptomatic patients with familial FSGS. Pediatr. Nephrol. 22, 1779–1784 (2007).
Wang, S. X. et al. Recurrence of nephrotic syndrome after transplantation in CNF is due to autoantibodies to nephrin. Exp. Nephrol. 9, 327–331 (2001).
Kuusniemi, A. M. et al. Plasma exchange and retransplantation in recurrent nephrosis of patients with congenital nephrotic syndrome of the Finnish type (NPHS1). Transplantation 83, 1316–1323 (2007).
Benfield, M. R., McDonald, R. A., Bartosh, S., Ho, P. L. & Harmon, W. Changing trends in pediatric transplantation: 2001 Annual Report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr. Transplant. 7, 321–335 (2003).
Callis, L., Vila, A., Carrera, M. & Nieto, J. Long-term effects of cyclosporine A in Alport's syndrome. Kidney Int. 55, 1051–1056 (1999).
Chen, D. et al. Cyclosporine A slows the progressive renal disease of Alport syndrome (X-linked hereditary nephritis): results from a canine model. J. Am. Soc. Nephrol. 14, 690–698 (2003).
Gross, O. et al. Antifibrotic, nephroprotective potential of ACE-inhibitor vs AT1 antagonist in a murine model of renal fibrosis. Nephrol. Dial. Transplant. 19, 1716–1723 (2004).
Grodecki, K. M. et al. Treatment of X-linked hereditary nephritis in Samoyed dogs with angiotensin converting enzyme (ACE) inhibitor. J. Comp. Pathol. 117, 209–225 (1997).
Gross, O. et al. Preemptive ramipril therapy delays renal failure and reduces renal fibrosis in COL4A3-knockout mice with Alport syndrome. Kidney Int. 63, 438–446 (2003).
Ninichuk, V. et al. Delayed chemokine receptor 1 blockade prolongs survival in collagen 4A3-deficient mice with Alport disease. J. Am. Soc. Nephrol. 16, 977–985 (2005).
Katayama, K. et al. Irradiation prolongs survival of Alport mice. J. Am. Soc. Nephrol. 19, 1692–1700 (2008).
LeBleu, V. et al. Stem cell therapies benefit Alport syndrome. J. Am. Soc. Nephrol. 20, 2359–2370 (2009).
Gross, O. et al. Stem cell therapy for Alport syndrome: the hope beyond the hype. Nephrol. Dial. Transplant. 24, 731–734 (2009).
LeBleu, V. S. & Kalluri, R. Stem cell-based therapy for glomerular diseases: an evolving concept. J. Am. Soc. Nephrol. 19, 1621–1623 (2008).
Wong, C. J. & Rogers, I. Cell therapy for Alport syndrome. J. Am. Soc. Nephrol. 20, 2279–2281 (2009).
Schiffmann, R. et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA 285, 2743–2749 (2001).
Eng, C. M. et al. Safety and efficacy of recombinant human alpha-galactosidase A—replacement therapy in Fabry's disease. N. Engl. J. Med. 345, 9–16 (2001).
Mehta, A. et al. Enzyme replacement therapy with agalsidase alfa in patients with Fabry's disease: an analysis of registry data. Lancet 374, 1986–1996 (2009).
Malina, M., Cinek, O., Janda, J. & Seeman, T. Partial remission with cyclosporine A in a patient with nephrotic syndrome due to NPHS2 mutation. Pediatr. Nephrol. 24, 2051–2053 (2009).
Gellermann, J., Stefanidis, C. J., Mitsioni, A. & Querfeld, U. Successful treatment of steroid-resistant nephrotic syndrome associated with WT1 mutations. Pediatr. Nephrol. 25, 1285–1289 (2010).
Lawless, M. W., Mankan, A. K., Gray, S. G. & Norris, S. Endoplasmic reticulum stress—a double edged sword for Z alpha-1 antitrypsin deficiency hepatoxicity. Int. J. Biochem. Cell Biol. 40, 1403–1414 (2008).
Inagi, R. Endoplasmic reticulum stress as a progression factor for kidney injury. Curr. Opin. Pharmacol. 10, 156–165 (2010).
Liu, X. L. et al. Defective trafficking of nephrin missense mutants rescued by a chemical chaperone. J. Am. Soc. Nephrol. 15, 1731–1738 (2004).
Acknowledgements
The authors' work described in this Review was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (19590939 to RI) and a grant from The Kidney Foundation, Japan (JKFB09-2 to RI). We are grateful to Masaomi Nangaku, MD, PhD, of Tokyo University School of Medicine, for critically reviewing the manuscript.
Author information
Authors and Affiliations
Contributions
C.-K. Chiang and R. Inagi contributed equally to researching data for the article, discussing content, writing, and reviewing/editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chiang, CK., Inagi, R. Glomerular diseases: genetic causes and future therapeutics. Nat Rev Nephrol 6, 539–554 (2010). https://doi.org/10.1038/nrneph.2010.103
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2010.103
This article is cited by
-
Successful production of genome-edited rats by the rGONAD method
BMC Biotechnology (2018)
-
The genetics of atypical hemolytic uremic syndrome
Medizinische Genetik (2018)
-
Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases
Nature Reviews Nephrology (2017)
-
RNA-seq of serial kidney biopsies obtained during progression of chronic kidney disease from dogs with X-linked hereditary nephropathy
Scientific Reports (2017)
-
Angiotensin II induces endoplasmic reticulum stress in podocyte, which would be further augmented by PI3-kinase inhibition
Clinical Hypertension (2015)